Trial Results: Journal of Clinical Oncology Publishes Final Results of E3311 Phase II Trial for Head and Neck Cancer
January 26, 2022
Trial Spotlight: Jarushka Naidoo on the EAQ172 Trial for Steroid-Refractory Pneumonitis
January 26, 2022Institution Spotlight: Georgia NCORP
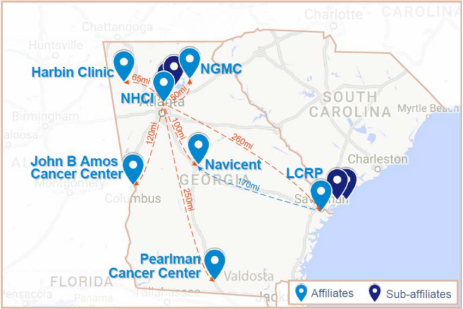

The Georgia National Cancer Institute (NCI) Community Oncology Research Program (Georgia NCORP or GA NCORP) represents a collaboration among Northside Hospital Cancer Institute in Atlanta, the Nancy N. and J.C. Lewis Cancer & Research Pavilion at St. Joseph’s/Candler in Savannah, and the Georgia Center for Oncology Research and Education (Georgia CORE). Northside Hospital, Inc. is the Grantee, providing the administrative oversight and financial accountability for GA NCORP.
Georgia CORE provides network affiliation for five contracted healthcare organizations across Georgia: Northeast Georgia Medical Center (Gainesville), Atrium Health Navicent (Macon), John B. Amos Cancer Center (Columbus), Pearlman Cancer Center (Valdosta), and Harbin Clinic (Rome). In total, the Georgia NCORP currently comprises 20 Affiliates and Sub-Affiliates.
With over 100 oncology clinical providers in 41 locations throughout the state, the Georgia NCORP partnership is poised to provide more Georgia residents living in both urban and rural areas with improved access to an expanded portfolio of state-of-the-art cancer prevention, screening, treatment, and post-treatment clinical trials, as well as cancer care delivery research. This community-based approach has the potential to remove barriers to accessing care, reduce cancer risk and incidence, and improve cancer care outcomes in Georgia.
Our dedicated team is successfully collaborating with the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) on several successful trials. Throughout our longstanding relationship that began in 1993, GA NCORP has consistently accrued almost 50% of its clinical trial participants to ECOG-ACRIN studies. Our team’s dedication and commitment are well displayed in our accruals, as well as our continuing leadership efforts in audits and membership across ECOG-ACRIN’s many scientific and administrative committees. Dr. Howard Zaren (see below) participates in Group governance/leadership by representing the Georgia NCORP on the ECOG-ACRIN Principal Investigator Committee.
Although recruitment is challenging during the pandemic, our group is collectively contributing at a high level to the TMIST (EA1151) breast cancer screening study—with over 1,550 women enrolled to date. A few other high-accruing studies include:
- NCI-MATCH (EAY131) - Molecular Analysis for Therapy Choice – n = 137
- EAZ171 - Prospective Validation Trial of Taxane Therapy (Docetaxel or Weekly Paclitaxel) and Risk of Chemotherapy-Induced Peripheral Neuropathy in African American Women – n = 23
- EA5163 / S1709 / INSIGNA - A Randomized, Phase III Study of First-line Immunotherapy Alone or in Combination with Chemotherapy in Induction/Maintenance or Postprogression in Advanced Non-squamous Non-Small Cell Lung Cancer (NSCLC) with Immunobiomarker SIGNature-Driven Analysis – n = 21
- EROS (E1Q11): Engendering Reproductive Health within Oncologic Survivorship – n = 17
- EAQ171CD - Implementing a Virtual Tobacco Treatment in Community Oncology Practices: "Smoke-Free Support Study 2.0" – n = 11
The population we serve is racially and socioeconomically diverse and includes rural patients. We seek to enroll diverse participants in our NCORP trials, with minorities representing over 23% of our patient accruals.
Our co-principal investigators for the Georgia NCORP share their personal perspectives below.
Guilherme Cantuaria, MD, PhD – Co-Principal Investigator (Northside Hospital Cancer Institute)
 As chairman of Northside Hospital's Cancer Program, I have been successful in transforming our community cancer program to mirror an NCI-Designated Comprehensive Cancer Center program. The Northside Hospital health care system is one of Georgia’s leading health care providers, with five acute-care hospitals in Atlanta, Canton, Cumming, Duluth, and Lawrenceville, and more than 250 outpatient locations across the state. The Northside Hospital Cancer Institute (NHCI) has a volume of over 10,000 newly-diagnosed cancers per year. My efforts have led to the development of a "hybrid model" that blends the best of a community cancer program, such as patient volume and satisfaction, with the best of an academic cancer center, such as integrated care and research. I have experience in cancer research in the community setting, from basic and translational research to clinical trials. I also have experience in cancer care delivery research, including research focused on reducing cancer disparities. I currently serve as chair of the Gynecologic Oncology Steering Committee at the NHCI.
As chairman of Northside Hospital's Cancer Program, I have been successful in transforming our community cancer program to mirror an NCI-Designated Comprehensive Cancer Center program. The Northside Hospital health care system is one of Georgia’s leading health care providers, with five acute-care hospitals in Atlanta, Canton, Cumming, Duluth, and Lawrenceville, and more than 250 outpatient locations across the state. The Northside Hospital Cancer Institute (NHCI) has a volume of over 10,000 newly-diagnosed cancers per year. My efforts have led to the development of a "hybrid model" that blends the best of a community cancer program, such as patient volume and satisfaction, with the best of an academic cancer center, such as integrated care and research. I have experience in cancer research in the community setting, from basic and translational research to clinical trials. I also have experience in cancer care delivery research, including research focused on reducing cancer disparities. I currently serve as chair of the Gynecologic Oncology Steering Committee at the NHCI.
Howard A. Zaren, MD, FACS – Co-Principal Investigator (Nancy N. and J.C. Lewis Cancer & Research Pavilion at St. Joseph’s/Candler)
 Promoting the advancement of clinical trial accruals and access to clinical trials has been a career priority for me, specifically in regard to minorities, medically underserved, and elderly communities. Given my tenure as the principal investigator (PI) for a Minority-Based Community Clinical Oncology Program (MBCCOP) at John H. Stroger, Jr., Hospital of Cook County in Chicago, a member of ECOG-ACRIN for over 30 years, and currently as the PI for the SJC/LCRP National Community Cancer Centers Program (NCCCP), I am dedicated to the NCORP opportunity. Over the last few years, I have had the opportunity to mentor a young investigator, Ashlesha Patel, MD, MPH (Stroger/Cook County Hospital), in advancing the EROS oncofertility trial (E1Q11) by ECOG-ACRIN which, in my opinion, will further cancer care delivery research.
Promoting the advancement of clinical trial accruals and access to clinical trials has been a career priority for me, specifically in regard to minorities, medically underserved, and elderly communities. Given my tenure as the principal investigator (PI) for a Minority-Based Community Clinical Oncology Program (MBCCOP) at John H. Stroger, Jr., Hospital of Cook County in Chicago, a member of ECOG-ACRIN for over 30 years, and currently as the PI for the SJC/LCRP National Community Cancer Centers Program (NCCCP), I am dedicated to the NCORP opportunity. Over the last few years, I have had the opportunity to mentor a young investigator, Ashlesha Patel, MD, MPH (Stroger/Cook County Hospital), in advancing the EROS oncofertility trial (E1Q11) by ECOG-ACRIN which, in my opinion, will further cancer care delivery research.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
