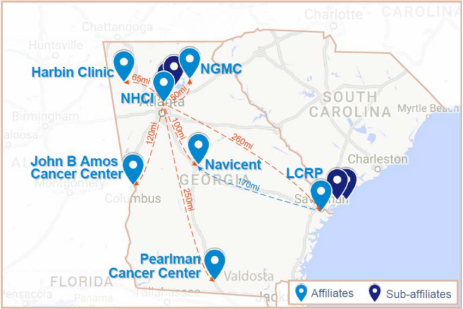
Institution Spotlight: Georgia NCORP
January 26, 2022
Come Again? Communicating Clearly About Risk of Recurrence After Early-Stage Breast Cancer
January 26, 2022Trial Spotlight: Jarushka Naidoo on the EAQ172 Trial for Steroid-Refractory Pneumonitis

Asian senior ill female have a cough and sore throat. Causes of cough include common cold, flu, respiratory tract infection, pneumonia, bronchitis, allergy or asthma. Elderly health care concept.
Optimizing Immunosuppression for Steroid-Refractory Anti-PD-1/PD-L1 Pneumonitis EGFR-Mutant Non-Small Cell Lung Cancer (NSCLC)
 By Jarushka Naidoo, MB BCH, MHS
By Jarushka Naidoo, MB BCH, MHS
Immunotherapy with immune checkpoint inhibitors can cause a range of immunologic toxicities, including inflammation of the lungs or pneumonitis. Pneumonitis is a potentially fatal toxicity of anti-PD-1/PD-L1 checkpoint inhibitors, and the condition is more common and severe in patients with lung cancer. It occurs in 2%-5% of patients receiving monotherapy, and up to 10% of those receiving combination therapy. While most cases of pneumonitis respond well to corticosteroid treatment, unfortunately 20% of cases are not responsive. In this situation, there is no clear optimal immunosuppressive treatment with proven benefit.
In order to identify which immunosuppressive treatment may be best for patients whose pneumonitis does not benefit from corticosteroids, we developed clinical trial EAQ172. EAQ172 is a randomized study comparing two of the most commonly used and potentially effective immunosuppressive treatments: infliximab versus intravenous immunoglobulin. The results of EAQ172 have the potential to make an immediate impact on clinical practice. The findings may advance the clinical care of patients by identifying an efficacious treatment for steroid-refractory pneumonitis.
This phase II trial is accepting patients with any solid tumor or hematologic malignancy, and they may have received any number of lines of prior systemic therapy. To qualify for study enrollment, patients must have had a comprehensive assessment to rule out infection (blood, sputum culture, and viral swabs) as well as a pathogen negative bronchoscopy within four weeks of enrollment. This therefore requires planning ahead of time, and partnership with pulmonary physicians and a bronchoscopist. In this study, we are also examining the radiographic features of pneumonitis using CT scans and the immunologic features using blood-based studies and any surplus fluid taken at the time of bronchoscopy. Our goal is to identify biomarkers of pneumonitis that could allow for risk stratification or potential prophylactic intervention in future studies.
EAQ172 is open and enrolling through the National Cancer Institute’s National Clinical Trials Network (NCTN). Medical oncology and pulmonary medicine co-principal investigators are required. Please join us!
Learn more about the EAQ172 trial at ecog-acrin.org.
Dr. Naidoo (Beaumont Hospital Dublin and Johns Hopkins University/Sidney Kimmel Cancer Center) is the study chair for this trial.
The study co-chairs are Sheetal Kircher, MD (Northwestern University), Mizuki Nishino, MD (Dana-Farber Cancer Institute), and Kathleen Yost, MD (Cancer Research Consortium of West Michigan NCORP).
NCTN group study champions are Josephine Feliciano, MD for Alliance (Johns Hopkins University/Sidney Kimmel Cancer Center), Dr. Naidoo for NRG Oncology, and David Page, MD for SWOG (Providence Cancer Center).
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
