
News in Brief, January 2022
January 26, 2022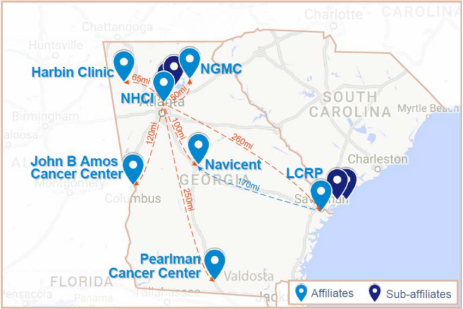
Institution Spotlight: Georgia NCORP
January 26, 2022Trial Results: Journal of Clinical Oncology Publishes Final Results of E3311 Phase II Trial for Head and Neck Cancer
Trial E3311 builds a strong case for the benefits and safety of transoral surgery and low-dose radiation in patients at medium risk for recurrence
Low-dose radiation at 50 Gy without chemotherapy following transoral surgery (TOS) led to very high survival and outstanding quality of life in patients with human papillomavirus-positive (HPV+) throat cancer and at medium risk for recurrence. The Journal of Clinical Oncology has published the final results of the randomized phase II trial E3311 showing that 94.9% of such patients were alive and disease-free three years later and had an excellent quality of life after this less intense treatment.
The approach preserved patients' swallowing and voice functions and spared them unnecessary short-term toxicities. The trial continues to follow patients to measure long-term survival and quality of life over five years.
 “Postoperative radiation therapy at 50 Gy without chemotherapy appears sufficient for intermediate-risk patients – those with uninvolved surgical margins, less than five involved nodes, and less than 1mm extranodal extension,” said senior author Barbara A. Burtness, MD (Yale Cancer Center), pictured.
“Postoperative radiation therapy at 50 Gy without chemotherapy appears sufficient for intermediate-risk patients – those with uninvolved surgical margins, less than five involved nodes, and less than 1mm extranodal extension,” said senior author Barbara A. Burtness, MD (Yale Cancer Center), pictured.
In E3311, 359 participants with HPV-related oropharyngeal (throat) cancer all underwent transoral surgery and were assigned to treatment based on individual risk factors for recurrence. The intensity of any additional treatment they received was based on factors known to predict whether the cancer is likely to spread or return, such as the size of the original tumor, the extent of cancer in neck lymph nodes, and others.
Only high-risk patients were assigned to chemotherapy, along with usual high-dose radiation (66 Gy, Arm D). Patients at low risk were observed and received no additional treatment (Arm A). Patients at intermediate risk were randomized to one of two arms to receive radiation alone, both at doses lower than usual (Arm B, 50 Gy or Arm C, 60 GY).
 “The E3311 findings are building a strong case that usual postoperative high-dose radiation and chemotherapy may not be necessary for all patients,” said first author Robert L. Ferris, MD, PhD (UPMC Hillman Cancer Center in Pittsburgh, PA), pictured.
“The E3311 findings are building a strong case that usual postoperative high-dose radiation and chemotherapy may not be necessary for all patients,” said first author Robert L. Ferris, MD, PhD (UPMC Hillman Cancer Center in Pittsburgh, PA), pictured.
After three years of follow-up, the E3311 trial showed two-year progression-free survival rates above 90% across all four groups: 96.9% for Arm A; 94.9% for Arm B; 96% for Arm C; and 90.7% for Arm D. Between Arms B and C, the progression-free survival rates were statistically the same, a strong indicator that radiation alone at 50 Gy was safe and effective for patients at intermediate risk.
This trial sets surgeon credentialing and quality assurance standards for future studies
E3311 is the first multi-center study of TOS in head and neck cancer. Surgeons and patients widely favor its organ-preservation approach. The trial collected prospective, multi-institutional data with meticulous and ongoing evaluation of surgeon expertise. There was a low incidence of positive margins and minimal oropharyngeal bleeding from TOS. This high level of surgical quality assurance demonstrated that the procedure could be performed safely.
The surgeon credentialing and quality assurance processes developed to support the E3311 trial provide standards for future transoral head and neck surgical oncology trials and improve their validity (Ferris RL, Burtness BA. Oral Oncol. November 2020).
E3311 provides quality of life data from the patients’ own perspectives
This trial used validated patient-reported outcome (PRO) scoring. Although researchers observed a consistent decline in quality of life and swallowing scores during treatment, these recovered to baseline in Arms A, B, and C. Scores following treatment in Arm D were slightly lower than baseline. Quality of life is important to measure because, in general, patients with HPV-related throat cancer experience profound, acute decreases in physical functioning and quality of life from open surgery, chemotherapy, and radiation treatments.
Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311)
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
