
Institution Spotlight: Penn State Cancer Institute
August 16, 2022
NCI-MATCH precision medicine trial leads to FDA drug approval for BRAF V600E-mutated tumors
August 16, 2022Trial Spotlight: Neha Vapiwala on the EA8191/INDICATE Study for Prostate Cancer
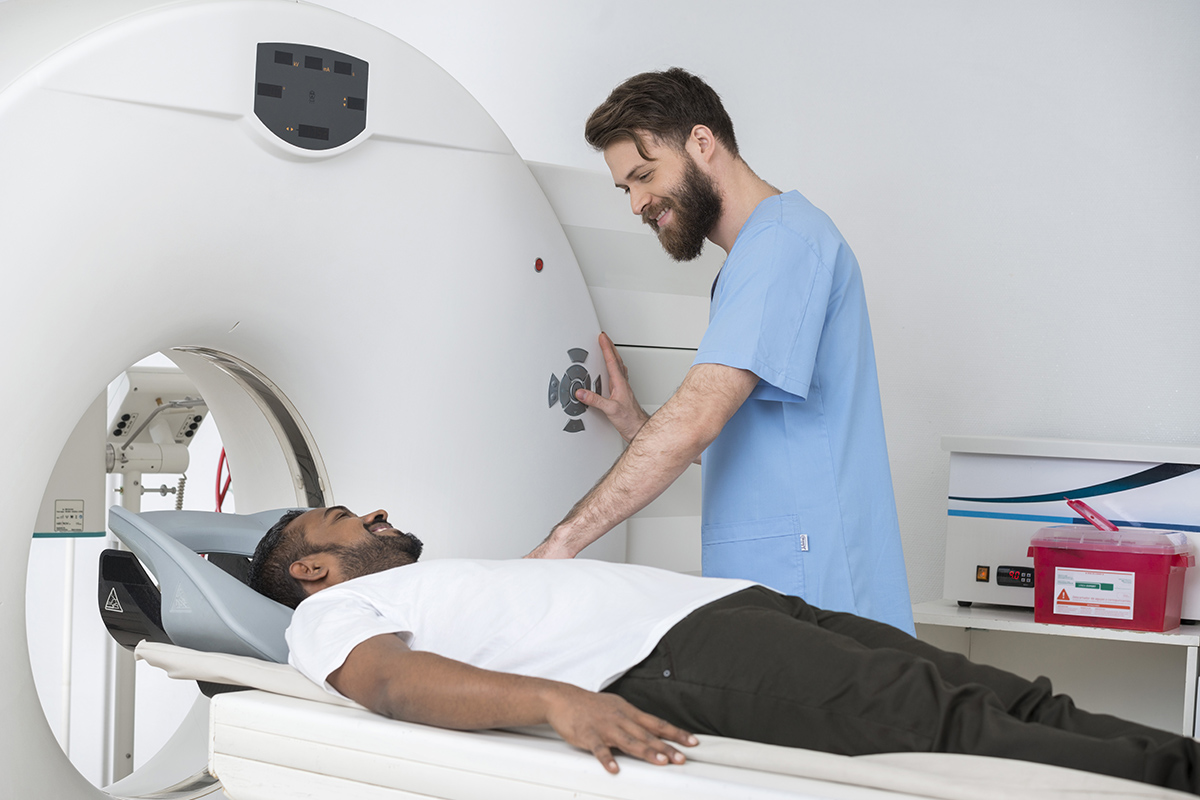
Phase III Study of Local or Systemic Therapy INtensification DIrected by PET in Prostate CAncer Patients with Post-ProstaTEctomy Biochemical Recurrence (INDICATE)
 By Neha Vapiwala, MD
By Neha Vapiwala, MD
Prostate cancer is the second most common cancer and the second leading cause of cancer-related death for men in the United States. Although radical prostatectomy is generally effective, up to 40% of patients may develop biochemical recurrence within 10 years of surgery. When this occurs, the usual standard-of-care (SOC) approach is radiation therapy (RT) to the prostate bed and pelvic nodes plus short-term (i.e., 6 months) androgen deprivation therapy (STAD). However, even after this salvage approach, some patients experience a second biochemical recurrence, raising questions about which subgroups of patients may harbor micrometastatic disease and which patients may benefit from treatment intensification. Improved imaging capabilities that can detect the site(s) of recurrence may help answer these questions and allow for tailored, risk-adapted approaches for patients when they first present with biochemical recurrence.
The phase III EA8191/INDICATE study, which opened in October 2020, is using PET/CT imaging to guide treatment for patients with post-prostatectomy biochemical recurrence. PET imaging is more effective at detecting small-volume metastatic disease compared to conventional imaging modalities such as MRI, CT, and bone scans. Thus, PET is being used by many clinicians to justify treatment modification, omission, or intensification, but without high-level evidence that clinical decisions based solely on the PET scan actually result in better patient outcomes.
There are two primary objectives for this study:
- For patients without PET evidence of extra-pelvic metastases, to evaluate if the addition of enhanced systemic therapy (apalutamide) to standard-of-care STAD may improve progression-free survival (PFS)
- For patients with PET evidence of extra-pelvic metastases, to evaluate whether the addition of metastasis-directed RT to apalutamide and standard-of-care STAD may prolong PFS
Androgen hormones can contribute to the growth of prostate cancer cells. Apalutamide is used to help fight prostate cancer by blocking the use of androgens by the tumor cells.
This trial may help determine if using PET/CT results to deliver more tailored treatment (i.e., adding apalutamide, with or without metastasis-directed radiation therapy, to SOC treatment) works better than SOC treatment alone in patients with biochemical recurrence of prostate cancer.
This trial has numerous secondary and quality-of-life objectives.
A recent amendment to the trial (active as of June 22 and available on the CTSU) contains a few key changes that may help sites enroll more patients. They are outlined here:
- An increase in the number of radiotracers that may be used for the initial PET/CT scan, including FDA-approved PSMA PET tracers
- Additional options for the 6 months of standard-of-care STAD, including oral GnRh antagonist therapy
- An increase in the timeframe for permitted baseline conventional imaging scans (now 26 weeks prior to Step 0 registration)
- An update that radiotracer training for reading radiologists is no longer required but is still strongly recommended
Patients who enroll in the study will undergo an initial baseline SOC PET/CT to determine if they have extra-pelvic metastases. Those without metastases will be stratified based on the presence or absence of intra-pelvic nodes and then randomized to either Arm A or B. Those with metastases outside the pelvis will be stratified based on the number of extra-pelvic lesions (five or fewer vs. more than five) and then randomized to Arm C or D. The various approaches each arm represents are listed below.
- Arm A: SOC RT and STAD
- Arm B: SOC RT and STAD plus apalutamide
- Arm C: SOC RT and STAD plus apalutamide
- Arm D: SOC RT and STAD plus apalutamide and metastasis-directed RT
This trial has the potential to be practice-changing regardless of the outcomes. If the results show a PFS benefit with earlier use of apalutamide and/or use of metastasis-directed RT in the group with extra-pelvic disease, this will inform the use of systemic and/or local therapy intensification based on PET findings alone to provide clinical benefit to patients. If the study does not find a PFS benefit with the experimental arm in either or both of the two randomizations, the findings will inform treatment guidelines accordingly and caution against routine off-label use of these intensification treatment strategies.
Patients are eligible for this study if they have biochemical recurrence after radical prostatectomy, with rising prostate-specific antigen levels. They must also be negative or equivocal for extra-pelvic metastases by conventional imaging modalities within 26 weeks prior to registration. Please see the protocol for full eligibility details.
Learn more about EA8191/INDICATE at ecog-acrin.org.
Dr. Vapiwala (University of Pennsylvania/Abramson Cancer Center) is the study chair for this trial.
The study co-chairs for imaging, medical oncology, and quality of life are Steve Cho, MD (University of Wisconsin/Carbone Cancer Center), Christos Kyriakopoulos, MD (University of Wisconsin/Carbone Cancer Center), and Alicia Morgans, MD (Dana-Farber/Harvard Cancer Center), respectively.
The NCTN group study champions are Rana McKay, MD (University of California San Diego/Moores Cancer Center) for Alliance, Bridget Koontz, MD (GenesisCare) for NRG Oncology, and Evan Yu, MD (University of Washington/Fred Hutchinson Cancer Research Center) for SWOG.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
