Trial Spotlight: Neha Vapiwala on the EA8191/INDICATE Study for Prostate Cancer
August 16, 2022
From the Co-Chairs, August 2022
August 16, 2022NCI-MATCH precision medicine trial leads to FDA drug approval for BRAF V600E-mutated tumors
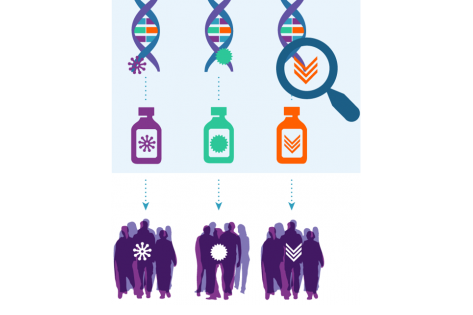
Dabrafenib/trametinib is the first and only BRAF/MEK inhibitor combination to be approved with a tumor-agnostic indication for solid tumors carrying the BRAF V600E mutation
A U.S. FDA drug approval has emerged from the groundbreaking NCI-MATCH precision medicine cancer trial. Based on data from Arm H of NCI-MATCH and two other clinical trials, the FDA granted accelerated approval on June 23, 2022, to dabrafenib plus trametinib for the treatment of adult and pediatric patients aged six years and older with unresectable or metastatic solid tumors harboring a BRAF V600E mutation whose disease has progressed following prior treatment and who have no satisfactory alternative treatment options. The ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) is co-leading the NCI-MATCH trial with the National Cancer Institute (NCI), part of the National Institutes of Health.
 "The FDA approval illustrates the ability of NCI-MATCH and other precision medicine trials to be nimble in response to positive findings and to develop positive efficacy signals into studies that have the potential to change clinical practice," said ECOG-ACRIN Group Co-Chair Peter J. O'Dwyer, MD, who co-leads NCI-MATCH.
"The FDA approval illustrates the ability of NCI-MATCH and other precision medicine trials to be nimble in response to positive findings and to develop positive efficacy signals into studies that have the potential to change clinical practice," said ECOG-ACRIN Group Co-Chair Peter J. O'Dwyer, MD, who co-leads NCI-MATCH.
Dabrafenib/trametinib is the first and only BRAF/MEK inhibitor combination to be approved with a tumor-agnostic indication for solid tumors carrying the BRAF V600E mutation, which drives tumor growth in more than 20 different tumor types. It is the only BRAF/MEK inhibitor combination approved for use in pediatric patients. BRAF V600E is, by far, the most common type of BRAF mutation, accounting for up to 90% of BRAF-mutant cancers (Turski ML. Mol Cancer Ther. April 2016).
The FDA approval was based on clinical efficacy and safety demonstrated in NCI-MATCH Arm H, a single-arm phase II substudy (Salama AKS. J Clin Oncol. August 2020), and the phase II ROAR (Rare Oncology Agnostic Research) basket study (Wen PY. Lancet Oncol. January 2022). In these studies, dabrafenib plus trametinib resulted in overall response rates of up to 80% in patients with BRAF V600E solid tumors. An additional study (Study X2101) demonstrated the combination's clinical benefit and acceptable safety profile in pediatric patients.
 “These findings suggest most patients whose tumors harbor a BRAF mutation can likely derive benefit from BRAF/MEK inhibition,” said April K.S. Salama, MD (Duke University), pictured, who serves as study chair for Arm H. “Importantly, the FDA approval expands the potential treatments for a group of patients who historically have had very limited choices.”
“These findings suggest most patients whose tumors harbor a BRAF mutation can likely derive benefit from BRAF/MEK inhibition,” said April K.S. Salama, MD (Duke University), pictured, who serves as study chair for Arm H. “Importantly, the FDA approval expands the potential treatments for a group of patients who historically have had very limited choices.”
NCI-MATCH is winding down, but researchers are still analyzing data from its many arms, and basing the design of future precision oncology trials on what this pioneering initiative got right, and the challenges it faced. As we finalize the registration protocol for the ComboMATCH trial (scheduled to activate in Q4 2022), we are excited to have a new set of therapeutic arms with which to advance genomically-defined therapy.
For more on NCI-MATCH, read NCI-MATCH: The Blueprint for Future Precision Medicine Trials or learn more about the trial at ecog-acrin.org.
Questions? Send an email to the trial team.
NCI-MATCH is sponsored by the NCI, part of the National Institutes of Health. ECOG-ACRIN is co-leading the trial with the NCI. Other NCI-funded network groups are participating: Alliance for Clinical Trials in Oncology, Children’s Oncology Group, NRG Oncology, and SWOG Cancer Research Network.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
