
From the Co-Chairs, October 2021
October 19, 2021
Institution Spotlight: Rutgers Cancer Institute of New Jersey
November 16, 2021News in Brief, November 2021

Mitchell Schnall Re-Elected as Group Co-Chair
The Principal Investigator Committee of the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) re-elected Mitchell D. Schnall, MD, PhD, as the Group Co-Chair, a position he has held since the founding of the Group in 2012. He represents the diagnostic imaging-based disciplines. Dr. Schnall will serve in this role from 2021 through 2031. The election occurred on November 2, 2021, under the oversight of the Nominating Committee. Link to the media advisory to learn more about Dr. Schnall's priorities for the upcoming term.
Melissa Simon Discusses Importance of Diverse Representation in Clinical Trials
In a recent interview with The Cancer Letter, Melissa A. Simon, MD, MPH, chair of ECOG-ACRIN’s Health Equity Committee, shared her thoughts on the state of diversity in cancer research. She was joined by other National Clinical Trials Network leaders, including Charles D. Blanke, MD of SWOG Cancer Research Network and Douglas S. Hawkins, MD of Children’s Oncology Group.
“There is without doubt substantial benefit to diverse participation in trials—the data will ultimately be more representative of and applicable to all populations,” said Dr. Simon. “Given the USA’s evolving demography to be more diverse over the next two decades, it behooves FDA and clinical trials efforts to prioritize diversity and inclusion in all trials. This will take thoughtful intentionality and true partnered approaches to build trust with communities who have been traditionally excluded from research or abused by research in the past.”
Research Results
Breast Cancer Survivorship—This study examined fatigue, sleep disturbance, and trouble remembering in a group of women who had completed breast cancer treatment in the E2Z02 trial (the majority were continuing hormonal therapies). It identifies the characteristics of women with high/severe versus low symptoms. The authors hope that clinicians will use this analysis to screen for patients who may require increased care to mitigate poor outcomes and impairments in quality of life during survivorship. Whisenant MS. Cancer Nurs. September 2021 (e-pub)
Leukemia—Central nervous system (CNS) involvement in newly diagnosed acute myeloid leukemia (AML) patients is a rare complication of the disease and is associated with a poor prognosis. However, systematic data regarding outcomes are scarce. This retrospective study summarized 11 consecutive ECOG-ACRIN clinical trials for newly diagnosed AML patients. The authors found a low incidence (1.1%) of CNS involvement at diagnosis among 3240 patients. There were no significant differences in the complete remission rate or overall survival between patients with CNS involvement and those with extramedullary disease (EMD) or those with no EMD. The presence of CNS disease at diagnosis does not appear, in and of itself, to portend for a poor prognosis. Ganzel C. Blood Adv. October 2021
Mesothelioma—After 20 years of limited progress in treating mesothelioma, the PrE0505 phase II trial results provide a potential new strategy to extend survival. The trial found that concurrent durvalumab immunotherapy with standard chemotherapy has promising clinical activity in patients with previously untreated malignant pleural mesothelioma (MPM). In-depth molecular analyses of responding tumors provide new insights into patient selection. Forde P. Nat Med. November 2021 and Press Release
- The DREAM3R phase III trial currently enrolls patients in the United States, Australia, and New Zealand to evaluate the durvalumab-chemotherapy combination versus chemotherapy alone in patients with MPM. This study is also exploring the genomic characteristics of tumor response.
Prostate Cancer—The phase III CHAARTED trial (E3805) established upfront androgen-deprivation therapy (ADT) plus docetaxel as a standard for metastatic hormone-sensitive prostate cancer (mHSPC) based on meaningful improvement in overall survival. A new analysis demonstrates the utility of transcriptomic subtyping for the potential selection of patients for chemo-hormonal therapy. It also provides proof of concept for the possibility of biomarker-guided selection of established combination therapies. Hamid A. Ann Oncol. September 2021
Throat Cancer—Primary transoral surgery followed by low-dose radiation (50 Gy) without chemotherapy led to very high survival and outstanding quality of life in patients with human papillomavirus-positive (HPV+) throat cancer and at intermediate risk for recurrence. The randomized phase II trial E3311 found that 94.9% of patients were alive and disease-free three years later and with excellent quality of life from this less intense treatment. This is the first cooperative group trial of transoral surgery, and the first trial to study transoral surgery for treatment de-intensification. Ferris RL. J Clin Onc. October 2021 (e-pub)
Mentoring Program Committee Selects Investigators for Awards of Distinction
Two presenters from the 2021 Young Investigator Symposium received awards of distinction—an annual tradition. Caner Saygin, MD (University of Chicago) won the distinction award for clinical research for his presentation. Clonal hematopoiesis is a precursor lesion for acute lymphoblastic leukemia in adults. Tharakeswara Bathala, MD, MBBS (MD Anderson Cancer Center) secured the distinction award for translational research; Dr. Bathala’s presentation was titled Defining Diagnostic Criteria for Prostatic Ductal Adenocarcinoma on Multiparametric MRI. Meet the eight 2021 Young Investigator Symposium presenters.
ECOG-ACRIN Grants Meeting Attendance Awards to Fifteen Minority Trainees
Fifteen underrepresented minority trainees attended the Fall 2021 Group Meeting thanks to virtual meeting attendance awards. Minority trainees include students, residents, fellows, and early-career investigators. The program also includes other trainees focusing on minority health or health disparities regardless of race or ethnicity. See below for a list of the fall 2021 recipients.
| Danielle Atibalentja, MD, PhD Stanford Cancer Institute |
Jamari Jemison Tennessee State University |
| Chaun Cheaney Tennessee State University |
John Kim Tennessee State University |
| Moriah Cunningham Thomas Jefferson University |
Michele Ly Thomas Jefferson University |
| Kayla Davis Tennessee State University |
Elizabeth McDuffie Thomas Jefferson University |
| Natasha Dhawan, MD Norris Cotton Cancer Center |
Antonio Ocejo-Gallegos, MD University of Miami |
| Latese Evans Thomas Jefferson University |
Ammanuel Taye, MD University of Alabama |
| Zaria Foster Tennessee State University |
Ashton Terrell Tennessee State University |
| Dakim Gaines, MD, PhD Vanderbilt University |

Video: EA Leaders Reflect on the Pandemic, Lessons Learned, and Share Thanks
NCI-MATCH Continues to Enroll Patients
The study chairs for this trial are Alice Chen, MD (NCI) and Keith Flaherty, MD (ECOG-ACRIN). The study co-chairs are Lyndsay Harris, MD (NCI) and Peter O'Dwyer, MD (ECOG-ACRIN).
Investigators and research staff, please continue to keep the open treatment arms top-of-mind when considering treatment options for your patients. Important updates:
- NCI-MATCH eliminated the need for a formal referral letter from the laboratories, with activation of Addendum #29 last month
- Arm Z1M (LAG-3 expression with MMR deficiency) accrual is temporarily suspended and will resume after the study team processes an amendment with updated safety information
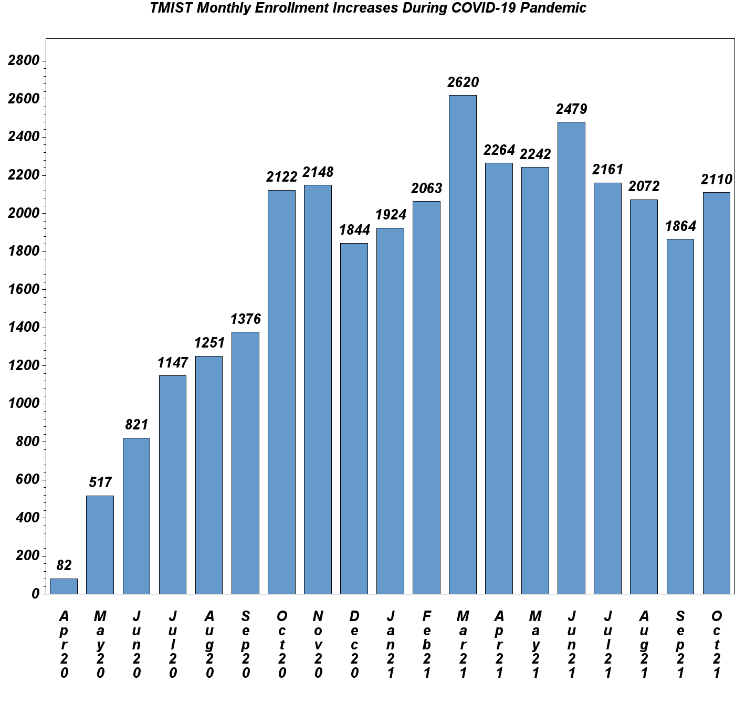
TMIST Growth Continues
The study chair for this trial is Etta Pisano, MD (Beth Israel Deaconess Medical Center, Harvard Medical School, and the American College of Radiology).
Amidst the ongoing pandemic, the TMIST breast cancer screening trial continues to enroll more patients than any other NIH-sponsored clinical trial. Monthly accrual remains strong (see chart below), and new sites continue to join the trial. Total accrual stands at 58,865 participants among 117 participating sites as of November 9, 2021. Important updates:
- Congratulations to Banner-University Medical Center Phoenix and University of Florida Health Science Center-Jacksonville for beginning enrollment!
- A study amendment activates on November 18 to modify TMIST to complete patient accrual and reach the primary study endpoint more quickly
- Participating sites have reported that some individuals eligible to join TMIST, especially women of color, could be declining to join the trial due to financial hardship and distress caused by the COVID-19 pandemic. As a result, a supplemental TMIST trial, EAQ201, will open soon and aims to enroll 1000 participants. Sites in the United States will offer a one-time survey to women who decline trial participation. The data will help study leaders to better understand this enrollment barrier.
- New sites may join this trial! If your site is interested in offering this trial to your constituents, email TMIST@acr.org to discuss the study requirements, reimbursement/payment structure, and how to start the application process.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
