
News in Brief, November 2021
November 16, 2021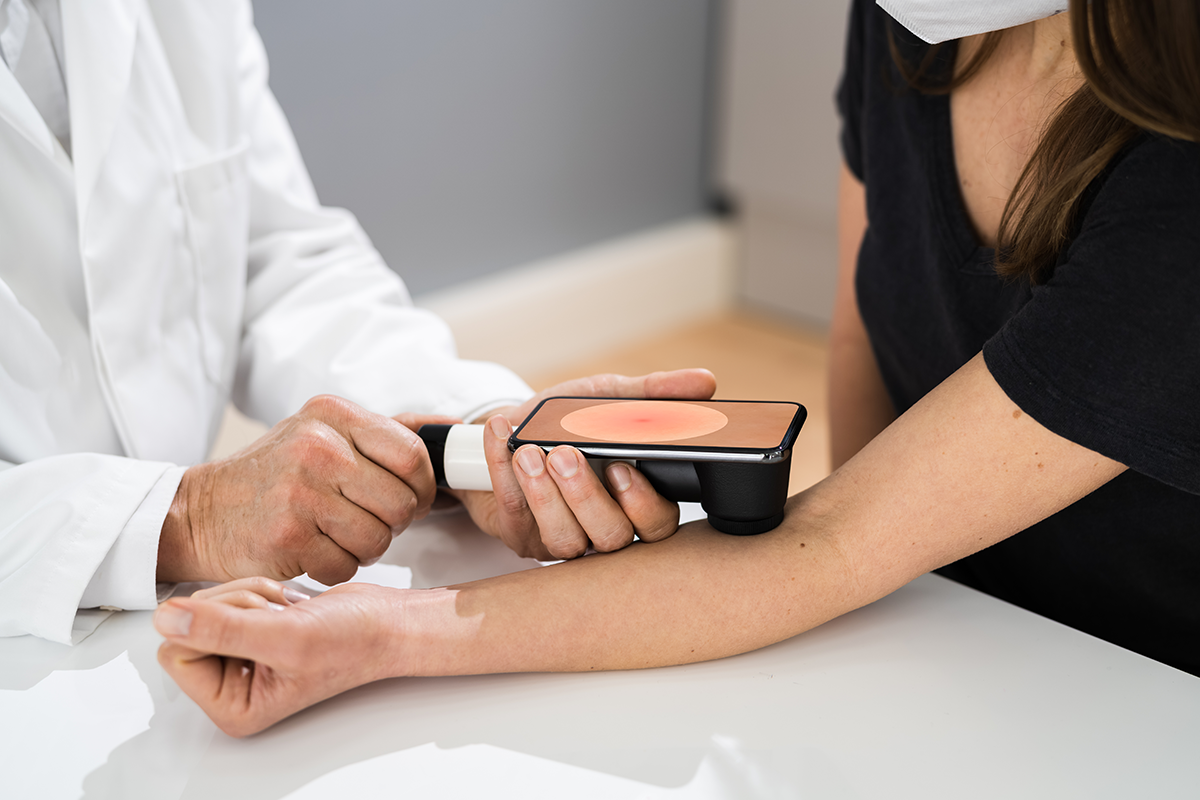
Trial Spotlight: Ravi Amaravadi on the EA6191/BAMM2 Trial for Melanoma
November 16, 2021Institution Spotlight: Rutgers Cancer Institute of New Jersey


By Howard S. Hochster, MD, FACP
Voting Member, ECOG-ACRIN Principal Investigator Committee
and
Associate Director for Clinical Research and Director of GI Oncology at Rutgers Cancer Institute of New Jersey; Director of Oncology Research at RWJBarnabas Health; and Distinguished Professor of Medicine at Rutgers Robert Wood Johnson Medical School
Rutgers Cancer Institute of New Jersey was established in 1993, became a National Cancer Institute (NCI)-designated Cancer Center in 1997, and achieved Comprehensive Cancer Center status in 2002. Originally called The Cancer Institute of New Jersey, it was integrated into Rutgers University on July 1, 2013, as an independent unit within the university. This integration was the result of the New Jersey Medical and Health Sciences Education Restructuring Act.
As New Jersey’s only NCI-designated Comprehensive Cancer Center, Rutgers Cancer Institute of New Jersey, in partnership with RWJBarnabas Health, has a team of internationally recognized physicians and researchers driven by a singular focus and mission – to help individuals fight cancer through research.
We provide the most advanced, comprehensive cancer care to adults and children at our main New Brunswick facility, Rutgers Cancer Institute of New Jersey at University Hospital in Newark, as well as through RWJBarnabas Health facilities across New Jersey. Further enhancing the delivery of services on the New Brunswick campus will be the opening of the new, freestanding Jack and Sheryl Morris Cancer Center – a 12-story, 510,000 square-foot cancer hospital expected in 2024.
Through the transformation of laboratory discoveries into clinical practice, we target cancer with precision medicine, immunotherapy, and clinical trials. This translational work is accomplished through relationships with many collaborators, including Princeton University. We operate under a “consortium cancer center” partnership as identified by the NCI, allowing for formal scientific and academic collaboration with Princeton University.
Research at Rutgers Cancer Institute is currently being conducted in areas of behavioral science, precision medicine, drug development and resistance, the relationship between cellular and genetic alterations and tumor development, cancer control and prevention, bioinformatics, and cancer genomics. And work on tumor immunology, autophagy, and cancer metabolism through our recently established Duncan and Nancy MacMillan Cancer Immunology and Metabolism Center of Excellence will further accelerate our work at the bench that will be translated into more effective and efficiently designed clinical trials – expanding our already extensive portfolio of Phase 1 investigator-initiated studies. This center will also focus on developing novel cellular therapies for patients with solid tumors, with the recent recruitment of Christian Hinrichs, MD, from the NCI.
There are several types of clinical trials currently underway at the Rutgers Cancer Institute, including treatment, prevention, screening, and behavioral/quality of life. The collaboration and infrastructure provided by our integrated care model with RWJBarnabas Health ensure we are able to offer these studies to our communities throughout the state. Rutgers Cancer Institute is also a UM1 site for trials funded by the NCI Experimental Therapeutics Clinical Trials Network (ETCTN), and we chair several of these important discovery trials. In addition, we are actively engaged with the Big Ten Cancer Research Consortium (BTCRC), where some of our investigators lead disease committees.
Rutgers Cancer Institute joined ECOG in 1996 and has since actively participated in numerous ECOG-ACRIN studies. Other Rutgers Cancer Institute colleagues are currently engaged in collaboration with ECOG-ACRIN including:
Edmund Lattime, PhD, is co-chair of the ECOG-ACRIN Laboratory Science and Pathology Committee; chair of the Laboratory Science Advisory Committee; and co-chair of the ECOG-ACRIN Immune Strategy Biomarker Subcommittee.
Andrew Evens, DO, MSc, is co-chair of the ECOG-ACRIN Lymphoma Committee; he led study E2408 which found that neither bortezomib added to bendamustine/rituximab (BR) induction nor lenalidomide added to rituximab maintenance immediately post-BR induction is recommended for untreated follicular lymphoma.
Kristen Spencer, DO, MPH, is a member of the recently formed ECOG-ACRIN Task Force on Advancement for Women; chair of the Developmental Therapeutics Working Group within the Gastrointestinal Cancer Committee; and site PI of EA2174: A Phase II/III Study of Peri-Operative Nivolumab and Ipilimumab in Patients with Locoregional Esophageal and Gastroesophageal Junction Adenocarcinoma.
Salma Jabbour, MD, is site PI of EA5181: Randomized Phase III Trial of MEDI4736 (durvalumab) as Concurrent and Consolidative Therapy or Consolidative Therapy Alone for Unresectable Stage 3 NSCLC.
Anupama Doraiswamy, MD, is site PI of EA9181: A Phase III Randomized Trial of Steroids + Tyrosine Kinase Inhibitor Induction with Chemotherapy or Blinatumomab for Newly Diagnosed BCR-ABL-positive Acute Lymphoblastic Leukemia in Adults.
Dale Schaar, MD, PhD, is site PI of EA9152: A Phase Ib/II Study of Venetoclax (ABT-199) in Combination with Liposomal Vincristine in Patients with Relapsed or Refractory T-cell or B-cell Acute Lymphoblastic Leukemia.
Mridula George, MD, is site PI of PrE0113: A Study to Assess Overall Response Rate by Inducing an Inflammatory Phenotype in Metastatic BReast cAnCEr With the Oncolytic Reovirus PeLareorEp in CombinaTion With Anti-PD-L1 Avelumab and Paclitaxel - BRACELET-1 Study.
Kevin David, MD, is site PI of PrE0405: Phase II Study of Bendamustine and Rituximab Plus Venetoclax in Untreated Mantle Cell Lymphoma Over 60 Years of Age.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
