
Virtual Spring 2021 Group Meeting: Tips and Highlights
April 27, 2021
From the Co-Chairs, April 2021
April 27, 2021Trial Spotlight: Search for patients intensifies with the opening of a new NCI-MATCH treatment arm
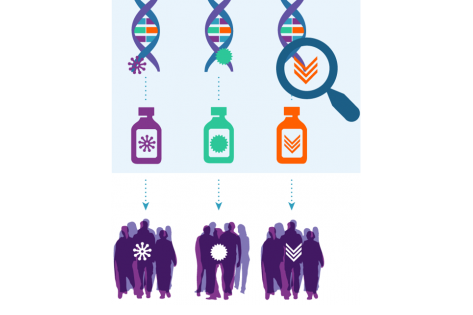
NCI-Molecular Analysis for Therapy Choice (NCI-MATCH)
The NCI-MATCH (EAY131) precision medicine trial is expanding this month with the opening of a new subprotocol. Arm Z1M is a single-arm phase II study of a two-drug combination, relatlimab and nivolumab, both immunotherapies. To be eligible, patients must have DNA mismatch repair deficiency (dMMR) on genomic testing and tumors that express the LAG-3 protein, a cancer immunotherapy target. The cancer must also have progressed on anti-PD-1/PD-L1 immunotherapy.
 In the following interview, Peter J. O'Dwyer, MD (pictured), ECOG-ACRIN Group Co-Chair and co-leader of the NCI-MATCH trial, shares progress on the trial and discusses its continuing relevance.
In the following interview, Peter J. O'Dwyer, MD (pictured), ECOG-ACRIN Group Co-Chair and co-leader of the NCI-MATCH trial, shares progress on the trial and discusses its continuing relevance.
What is the current status of NCI-MATCH?
The trial is ongoing and continuing to offer treatment options for patients. With the addition of Z1M, the open arms in NCI-MATCH address a range of 12 unique molecular targets and corresponding drugs that have evidence they work against that target. We urge physicians and staff at current sites to keep these arms top of mind when looking for treatment options for your patients. We certainly recognize and appreciate the extra time physicians and their staff are taking to find patients with these aberrations.
Can new sites join the trial?
Yes. We encourage more NCI-affiliated sites to open the trial. If your site is ordering standard testing from one or more of the NCI-designated labs to guide clinical care for your patients, then you already have the mechanism in place to identify patients. With several treatments in a single trial, participating oncologists have a range of treatment arms ready to offer when their patients' genomic test results match one or more of the tumor abnormalities. The patients we are seeking don't have many other opportunities. For community-based sites, NCI-MATCH provides many patients the ability to receive protocol treatment close to home.
Which patients does NCI-MATCH serve?
NCI-MATCH enrolls patients with advanced solid tumors, lymphomas, or myelomas that have progressed on standard treatment, or patients with rare types of cancer for which there is no standard treatment. Eligibility depends upon molecular abnormalities instead of the organ site of cancer. Tumor testing by an NCI-designated lab is the only pathway for patients at participating locations to enroll in the NCI-MATCH trial.What is the primary aim of MATCH?
NCI-MATCH is providing critical new information about possible cancer treatments. Each arm — and every patient who participates — is contributing valuable information on responsive versus unresponsive cancer types, especially in rare cancers where there are little or no data available. Overall, the trial aims to pick up signals of efficacy. Such discoveries could be eligible to move on to larger, more definitive trials.NCI-MATCH Subprotocols Open and Enrolling
| Molecular Target | Accrual a/o 4-18-21 |
Drug(s) | Arm |
|---|---|---|---|
| BRAF V600E or BRAF V600K mutations | 1/50 (Expanded) |
dabrafenib & trametinib | H |
| CDK4 or CDK6 amplification | 42/49 (Expanded) |
palbociclib | Z1C |
| cKIT mutations | 10/35 | sunitinib malate | V |
| EGFR activating mutations | 19/35 | afatinib | A |
| EGFR T790M | 11/35 | AZD9291 | E |
| LAG-3 expression and dMMR status | 0/35 | relatlimib & nivolumab | Z1M |
| MET amplification | 41/50 (Expanded) |
crizotinib | C1 |
| MET exon 14 deletion | 20/35 | crizotinib | C2 |
| mTOR, KEAP1, or NFE2L2 mutations | 26/35 | TAK-228 | L |
| NTRK fusions | 15/35 | larotrectinib | Z1E |
| PTEN loss without PIK3CA mutations | 20/35 | copanlisib | Z1G |
| TSC1 or TSC2 mutations | 44/49 (Expanded) |
TAK-228 | M |
Why do some treatment arms have higher accrual goals?
This is an important question, and the answer speaks, in large part, to how we are able to adjust the trial for patient opportunity. Generally, we aim to enroll 35 patients for each treatment arm. However, four of the 12 current subprotocols have additional slots ready and available, knowing that the labs will identify more potential candidates for these arms than those with less prevalent targets. The four expansion arms are targeting BRAF V600E or BRAF V600K mutations, CDK4 or CDK6 amplification, MET amplification, and TSC1 or TSC2 mutations.
A promising efficacy signal is also the reason for expanding Arm H. This arm investigates the selective BRAF inhibitor dabrafenib and the MEK1/2 inhibitor trametinib in patients whose tumors harbor BRAF V600 or BRAF V600K mutations. The initial cohort met its primary endpoint, with an objective response rate of 38% (P < .0001) in a mixed histology, pretreated cohort of 29 evaluable patients (Salama AKS. J Clin Oncol. 2020 Aug 06). We hope to identify 50 more patients for Arm H, to better define the broad applicability of the recently-published positive findings in the initial cohort.
How is the laboratory referral process working?
Foundation Medicine, Inc.
Caris Life Sciences®
Ashion Analytics, LLC
CellNetix Pathology and Laboratories
GenPath (BioReference Laboratories, Inc.)
NeoGenomics Laboratories, Inc.
Quest Diagnostics Inc.
Strata Oncology, Inc.
Tempus Labs, Inc.
The Jackson Laboratory
Are there other ways to find out about eligibility?
For patients who have test results from one of the participating laboratories, they or their oncologist may send an email directly to that lab to request a review of a genomic test. The ECOG-ACRIN website contains contact information for the NCI-designated commercial labs. We encourage physicians and patients to reach out directly to the labs. There is no need to contact the National Cancer Institute, the trial sponsor, or ECOG-ACRIN.
Is this patient referral process unique?
Yes and no. Molecular testing for cancer treatment selection is a common practice. Medicare and most insurance companies cover it. NCI-MATCH is the only trial to build an extensive network of testing laboratories, which is necessary to find patients for a study where molecular features determine eligibility. The NCI-designated lab network includes not only 11 commercial labs but also 17 academic labs. Generally, the designated academic labs are in cancer centers and identify potential trial candidates from their own patient populations.
NCI-MATCH is the largest precision medicine cancer clinical trial to date with 39 single-arm phase II treatment arms. Researchers are locating patients for the 12 remaining arms as the results are published or being analyzed for the other arms. The ECOG-ACRIN Cancer Research Group and the National Cancer Institute are co-leading NCI-MATCH, which is being conducted through the NCI National Clinical Trials Network.
Learn more about NCI-MATCH at ecog-acrin.org.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
