
From the Co-Chairs, December 2019/January 2020
January 14, 2020
News in Brief, February 2020
February 28, 2020Trial Spotlight: Nabil Saba on Study EA3161 for HPV Positive Head and Neck Cancer
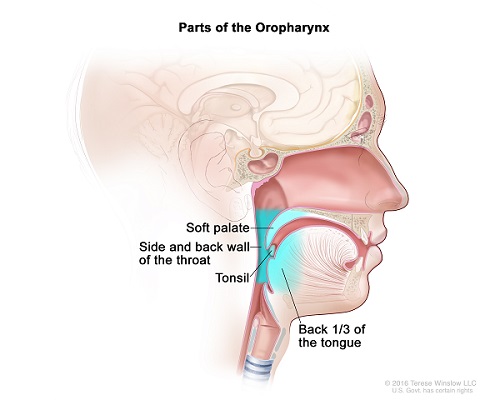
Created for the National Cancer Institute, http://www.cancer.gov
A Phase II/III Randomized Study of Maintenance Nivolumab versus Observation in Patients with Locally Advanced, Intermediate Risk Human Papilloma Virus (HPV) Positive Oropharyngeal Squamous Cell Carcinoma (OPSCC)

By Nabil F. Saba, MD
Despite the substantially better survival among patients with HPV-driven OPSCC compared to HPV-negative disease, patients with p-16 positive OPSCC with intermediate risk defined as T4 or N2-N3 (AJCCS) or smokers of more than 10- pack-years seem to be at the highest risk for death with an estimated 4-year OS of 680/o. Patients with T3N3 or T4N2- N3 are at especially high risk with a 4-year survival of 510/o. The clinical benefit from immune checkpoint inhibitors (ICI) in recurrent metastatic squamous cell carcinoma of the head and neck (SCCHN) may be reflected in a prolonged stabilization of disease, raising interest in employing ICI as a form of maintenance therapy.
Recent evidence indicated that nivolumab maintained function and improved symptoms in heavily pre-treated patients with SCCHN, making its use in a maintenance setting attractive. EA3161 is a phase II/III trial randomizing patients with intermediate-risk HPV-related OPSCC to maintenance nivolumab versus observation following definitive therapy with intensity-modulated radiation therapy (IMRT) and cisplatin. EA3161 will provide robust, biologically significant correlative studies in this patient population. As this study focuses on HPV-related disease, it also avoids intensification of concurrent therapy and relies instead on abrogating the risk of recurrence through a maintenance approach.
EA3161, a phase II trial, is assessing the efficacy of concurrent definitive therapy followed by nivolumab compared with concurrent definitive therapy followed by observation in terms of progression-free survival (PFS). EA3161 (phase III) is assessing the efficacy of concurrent definitive therapy followed by nivolumab compared with concurrent definitive therapy followed by observation in terms of overall survival (OS). Additional objectives include assessing the efficacy of nivolumab compared to observation in terms of relationship to baseline PD-L1 expression and the 12-week post-therapy FDG PET/CT.
Eligible patients must be at least 18 years of age with ECOG PS O or 1, and must have oropharynx cancer that is p16-positive by immunohistochemistry with smoking status: 10 pack-years, stage T1-2N2-N3 or T3-4N0-3 OR <10 pack-years, stage T4NO-N3 or T1-3N2-3.
Though it is quite probable that adding ICI to the backbone standard concurrent regimens will be proven effective for locally advanced SCCHN, the question of whether intensification specifically for the HPV-related OPSCC is the correct approach, and the degree to which this specific group will benefit from maintenance ICI will continue to be relevant. EA3161 is uniquely positioned to answer these important questions for a healthier group of patients with better prognosis yet the equivalent risk of distant metastases.
Learn more about study EA3161 at ecog-acrin.org.
Dr. Saba (Emory University, Winship Cancer Institute) is the lead investigator for this trial.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
