Institution Spotlight: Puerto Rico Minority Underserved NCORP
March 31, 2021
Now Enrolling: PrE0506 / DREAM3R for Patients with Malignant Pleural Mesothelioma
March 31, 2021Trial Spotlight: Amer Zeidan on the BLAST MRD CML 1 Study for Chronic Myeloid Leukemia

BLAST MRD CML 1 Trial: Blockade of PD-1 Added to Standard Therapy to Target Measurable Residual Disease (MRD) in Chronic Myeloid Leukemia (CML) - A Phase II Study of Adding the Anti-PD-1 Pembrolizumab to Tyrosine Kinase Inhibitors in Patients with CML and Persistently Detectable MRDn

By Amer M. Zeidan, MBBS
Chronic myeloid leukemia (CML) is driven by the activity of the oncogenic BCR-ABL1 tyrosine kinase, which can be effectively inhibited by tyrosine kinase inhibitors (TKIs), leading to prolonged overall and disease-free survival. However, disease can progress on TKI therapy—and even when it does not, lifelong treatment with TKIs can have substantial negative effects on patients’ quality of life and financial resources. Previous studies that assessed the safety of TKI discontinuation in CML patients in molecular remission demonstrated that TKIs can be discontinued in up to 45% of patients, without disease recurrence.
Programmed death receptor-1 (PD-1) is an inhibitory immune checkpoint receptor expressed by T-cells that can be inhibited by the anti-PD-1 monoclonal antibody pembrolizumab. Preclinical data have shown that PD-1 is highly expressed on CML-specific cytotoxic T-cells and PD-1 ligand (PD-L1) is present on CML cells. In murine CML models, the administration of anti-PD-L1 antibodies prolonged survival. Since these studies suggest disease relapse may be due to PD-1/PD-L1 mediated immune escape by CML cells, the BLAST MRD CML 1 trial will test the effects of adding pembrolizumab to TKI therapy. The study will assess safety, the rate of conversion to undetectable minimal residual disease (uMRD) and the rate of TKI discontinuation.
Patients may be eligible for BLAST MRD CML 1 if they have pathologically-confirmed CML and have achieved major molecular response but not uMRD at time of screening. They must also have received first, second, or third line TKI therapy with either dasatinib, imatinib, nilotinib, or bosutinib for at least two years prior to enrollment.
The primary endpoint of BLAST MRD CML 1 is to assess the proportion of CML patients on stable-dose TKI who convert to a state of uMRD during or within two years of initiating pembrolizumab therapy.
In this non-randomized design, all eligible study participants will enter Arm A, in which they continue their standard dose TKI therapy and receive pembrolizumab at 200 mg IV every 21 days for 18 cycles (approximately one year). Minimal residual disease (MRD) status will be assessed centrally by measuring the level of the BCR-ABL1 gene product in the blood every 4 cycles. If MRD becomes undetectable during Arm A, the patient will discontinue pembrolizumab after cycle 18 and continue on TKI therapy alone. If MRD remains undetectable for one year after the first negative assay, the patient will discontinue TKI therapy and remain in the study for follow-up. Otherwise, patients who remain MRD positive at the end of Arm A will switch to Arm B and continue pembrolizumab for approximately one more year (cycles 19-36), along with TKI therapy, with clinical follow-up thereafter.
Secondary endpoints include evaluating the proportion of CML patients who maintain uMRD status for six months and 12 months; the proportion of CML patients who discontinue TKI therapy after achieving uMRD; the proportion of patients who maintain uMRD off TKI therapy at two years after first determined uMRD; and the incidence of grade 3 or 4 immune related adverse events related to pembrolizumab treatment during the first two years after registration.
Learn more about the BLAST MRD CML 1 trial at ecog-acrin.org
Dr. Zeidan (Yale University) is the lead investigator for this trial.
| Why do some CML patients have an excellent response to therapy, while others have a poor response? TKI therapy has revolutionized the treatment of CML. However, the main two clinical questions in CML therapy are when to change therapy and when to stop therapy. We still do not know why some patients respond so well to TKI therapy and others respond so poorly. If we understood the biology and good versus poor responses, could we predict response before treatment, add new therapies for patients bound for a poor response, and change patient fate to that of a good responder? Using diagnostic samples from the ENESTnd study (which randomized chronic phase patients between nilotinib and imatinib), RNA sequencing was performed in 112 patients dichotomizing cases into good responders (BCR-ABL1 ≤10% IS at 3 and 6 months and ≤0.1% by 12 months) and poor responders (failure to meet these criteria). Both the top genes and pathways upregulated in the good responders were involved in the immune system. All of the top 20 pathways overexpressed in good responders involved immune regulation, a finding validated in an independent data set. Good responders had elevated PD-1 and PD-L1 compared to poor responders. This study emphasizes the importance of pretreatment adaptive immune response in treatment efficacy and suggests biological pathways that can be manipulated to improve response. The paper, entitled “Molecular response in newly diagnosed chronic myeloid leukemia in chronic phase: prediction modeling and pathway analysis” has been submitted for publication. – Jerald Radich, MD Fred Hutchinson Cancer Research Center BLAST MRD CML 1 Laboratory Chair 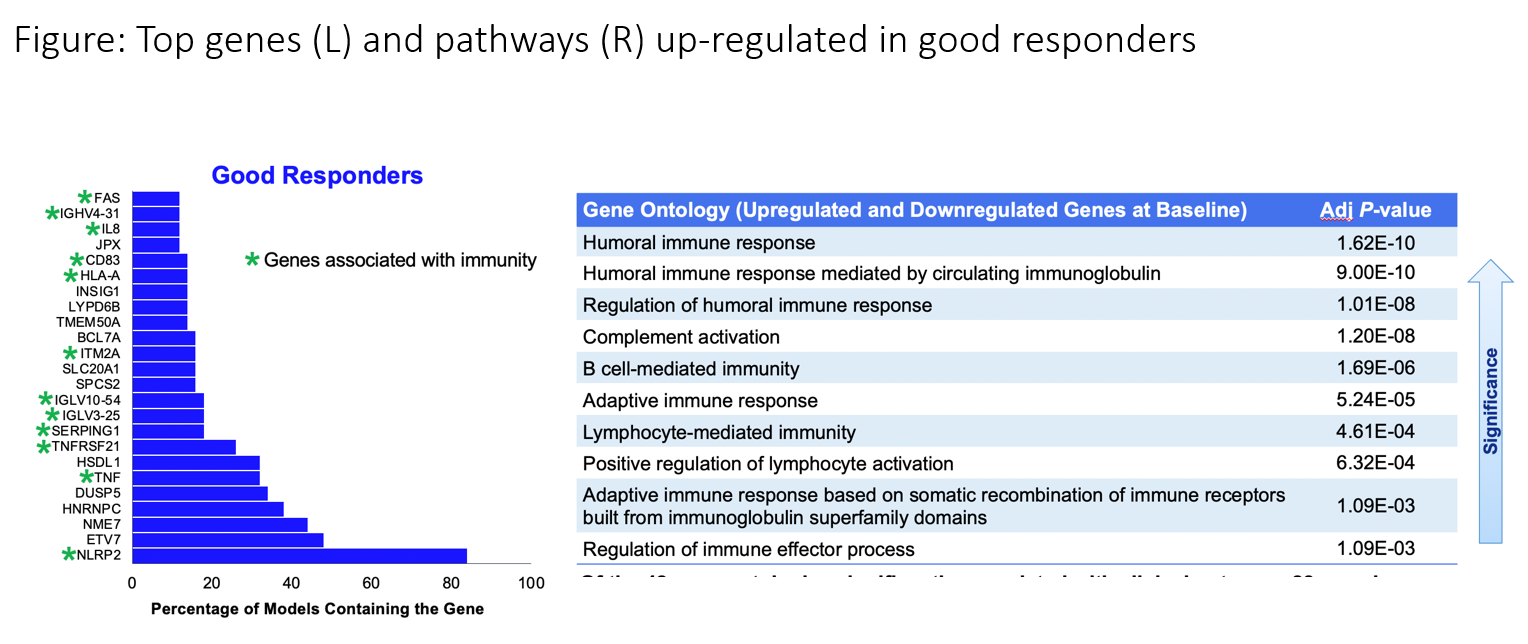 |
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
