
Trial Spotlight: Kim Reiss on the EA2192/APOLLO Study for Certain Patients with Pancreatic Cancer
April 19, 2023
From the Co-Chairs, April 2023
April 19, 2023Now Enrolling: ECOG-ACRIN Opens the ComboMATCH Patient Registration Trial as the Gateway to a Coordinated Set of Treatment Trials

EAY191: Molecular Analysis for Combination Therapy Choice (ComboMATCH)
The study chair for this trial is James M. Ford, MD (Stanford University/Stanford Cancer Institute). The co-chair is Funda Meric-Bernstam, MD (MD Anderson Cancer Center).
The ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) ComboMATCH patient registration trial (EAY191) is now open to enrollment as the gateway to a coordinated set of clinical trials to study cancer treatment directed by tumor biology. ComboMATCH is the latest precision medicine cancer initiative to be funded by the National Cancer Institute (NCI). Unlike other planned initiatives, ComboMATCH is based on principles of reliance on pre-clinical in vivo evidence. Through such principles, ComboMATCH researchers are discovering new drug combinations that show potential to overcome drug resistance to single-agent therapy or support the use of novel synergies to increase efficacy in treating patients with advanced cancers.1
The EAY191 registration trial, managed by ECOG-ACRIN, contains the rules for assigning patients to various treatment trials—and other guidelines for participating trial sites. As of this date, three treatment trials are open for patient enrollment. Seven additional studies will activate shortly. Each treatment protocol will evaluate a specific drug combination in a selected group of patients with locally advanced or advanced solid tumors. Each has its own research goals, patient eligibility criteria, and enrollment timeline.
Multiple researchers are leading each treatment trial, offering opportunities for both junior and senior investigators to work alongside translational researchers.
The first step for patients and physicians interested in a ComboMATCH treatment trial is enrolling in the patient registration trial through the oncologist. The registration trial eligibility criteria are as follows*:
- Locally advanced or metastatic solid tumors with measurable disease
- Must have progressed on at least one line of standard systemic therapy OR have a disease for which no standard treatment exists to prolong overall survival
- ECOG Performance Status (PS) grade 0-2 OR Lansky/Karnofsky PS ≥ 50%
- ≥ 18 years old: tumor amenable to minimal risk biopsy and undergo a tumor biopsy to obtain samples for research, OR confirm availability of an archival FFPE tumor tissue specimen collected within 12 months prior to registration, without a complete/partial response to any intervening therapy
- < 18 years old: confirm availability of an archival FFPE tumor tissue specimen for submission for research
*Review the complete eligibility details for the registration trial here.
During the registration trial, oncologists will review the test results, along with other clinical information, to determine if their patients are eligible for one of the treatment trials.
Molecular eligibility for the treatment trials will be determined using archival tumor tissue specimens analyzed by genetic testing laboratories designated by the NCI to participate in ComboMATCH. Physicians routinely order these tests to guide clinical care for their patients.
There are two ways in which patients will be identified for possible participation in a treatment trial. First, physicians at participating clinical sites may review their patient's test results to determine if they show any of the molecular alterations being investigated in ComboMATCH. Second, designated labs will inform oncologists at participating trial sites when patients' tumor test results show potential matches.
Nearly 40 commercial and academic labs are activating ComboMATCH on a rolling basis. A downloadable spreadsheet of the participating laboratories is available on the CTSU and is updated weekly. The information is also available at ecog-acrin.org.
Below is a visual of the registration and treatment trial process from the Study Flowchart (available at CTSU and ecog-acrin.org). Other educational materials are available here.
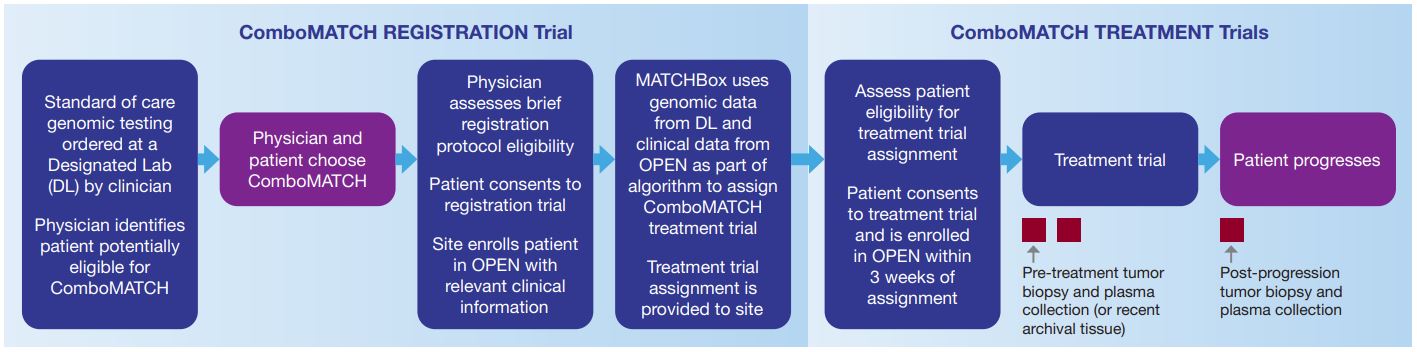
This information is intended for health care professionals and is not intended for use as patient educational material.
Patients matched to a trial will be asked to provide a pretreatment tumor biopsy specimen for genomic profiling.
ComboMATCH builds on the findings of NCI-MATCH which also paired patients with treatment based on their cancer's molecular abnormalities. NCI-MATCH was groundbreaking as one of the first and largest precision medicine cancer trials. The study demonstrated that targeting genetic changes in a tumor may be an effective way to treat cancer. It also showed that people with advanced cancer may benefit from tumor testing to help plan their treatment.
ComboMATCH vs. NCI-MATICH
- While NCI-MATCH was mainly comprised of single-arm studies, ComboMATCH will test combination therapies—either two targeted agents together or a targeted drug with chemotherapy
- NCI-MATCH was histology agnostic with selected tumor exclusions, while ComboMATCH has both histology-specific and histology-agnostic arms
- While NCI-MATCH consisted of single-arm studies, ComboMATCH utilizes single-arm as well as randomized designs
- NCI-MATCH had a separate, parallel Pediatric MATCH trial, whereas some ComboMATCH trials will include both children and adults within the same study
Learn more about ComboMATCH at ecog-acrin.org.
ComboMATCH is a large precision medicine initiative with a coordinated set of clinical trials evaluating novel drug combinations. The ComboMATCH patient registration trial (EAY191) is being led by the ECOG-ACRIN Cancer Research Group and National Cancer Institute; treatment trials are being led by the Alliance for Clinical Trials in Oncology, Children's Oncology Group, ECOG-ACRIN, NRG Oncology, and SWOG Cancer Research Network.
1. Meric-Bernstam F, Ford JM, O'Dwyer PJ, et al. National Cancer Institute Combination Therapy Platform Trial with Molecular Analysis for Therapy Choice (ComboMATCH). Clin Cancer Res 2023; OF1-OF11.↩
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
