
Trial Spotlight: Stephen Hodi on the EA6141 Study for Patients with Advanced Melanoma
April 9, 2024
From the Co-Chairs, April 2024
April 9, 2024Now Enrolling: EAF223/GABLE for Patients with Glioblastoma
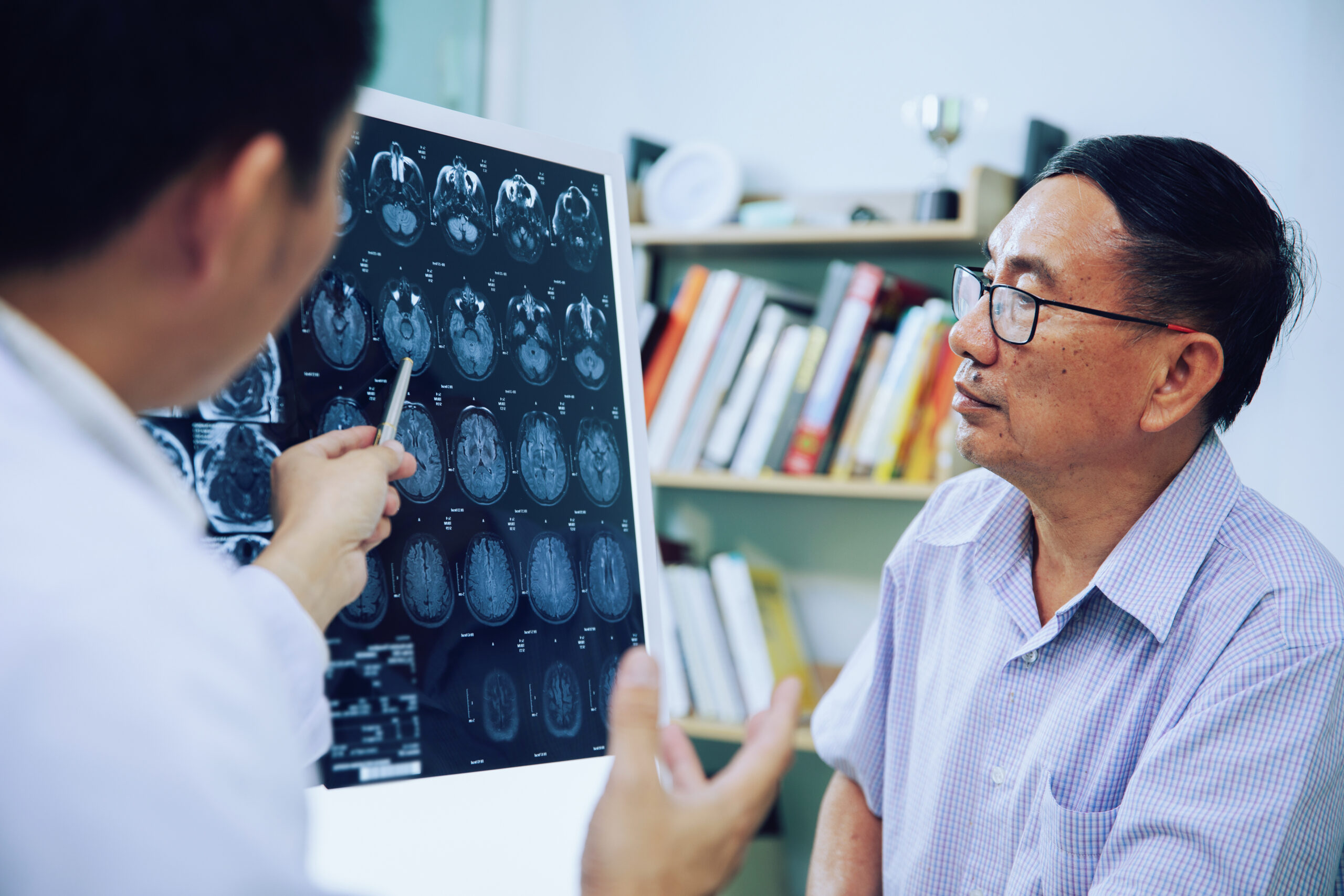
Phase II Glioblastoma Accelerated Biomarkers Learning Environment Trial (GABLE)
The study chair for this trial is Daniel Barboriak, MD (Duke University Medical Center). The study co-chairs are Kathleen Schmainda, PhD (Medical College of Wisconsin), Jonathan McConathy, MD, PhD (University of Alabama at Birmingham Cancer Center), and Lawrence Kleinberg, MD (Johns Hopkins University/Sidney Kimmel Cancer Center). The community co-chair is Jerrold L. Boxerman, MD, PhD (Rhode Island Hospital).
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most aggressive form of brain cancer. These tumors develop from supportive cells in the brain called glial cells, grow rapidly, and invade adjacent brain tissues. They are most common in adults over 50 and have a poor prognosis, with an average survival rate of around 14 months after diagnosis.
Treatment of these aggressive tumors is challenging. The standard treatment for a patient with newly diagnosed GBM – maximal safe surgical resection followed by radiation therapy and the use of a drug called temozolomide, which alkylates tumor DNA – has not changed in almost twenty years. Although this treatment has resulted in more patients living three years or longer after diagnosis, most patients do not survive this long, and the treatment is not curative.
Most commonly, contrast-enhanced MRI is used to evaluate tumor burden in these patients after the completion of radiation therapy. Unfortunately, MRI scans can give misleading results, particularly in the first three months after completion of radiation therapy. During this time period, it is not unusual for MRI scans to give the false impression that the tumor has increased in size. Unfortunately, because of this, oncologists are reluctant to treat patients for progression of GBM unless the appearance of tumor growth has been confirmed with follow-up MRI scans, which often occur more than three months after completion of radiation therapy. The failure of MRIs to accurately depict tumor burden early in the course of GBM treatment has resulted in patients having larger tumors at the time they are eventually treated, and these larger tumors may well be more challenging to treat.
EAF223/GABLE is a phase 2 imaging biomarker trial to evaluate whether novel biomarkers can accurately identify which patients will have better vs. worse survival outcomes early in their disease course. To be eligible for this study, patients must be 18 years of age or older, have a Karnofsky Performance Status score of at least 60, and be diagnosed with GBM based on WHO 2021 criteria. Patients must have completed surgery at the time of enrollment and be planning for standard-of-care treatment for GBM, including radiotherapy and temozolomide.
Patients enrolling in this trial will receive standardized MR scans including dynamic susceptibility contrast-enhanced perfusion imaging (DSC-MRI) as part of the routine imaging they would normally receive. If these scans show findings raising the suspicion of increasing tumor burden, patients will return to an imaging center for one additional scan, which is investigational.
Initially, this additional scan will be a PET scan using fluciclovine, an imaging agent that has already been FDA-approved for patients with prostate cancer. Later in the trial, after the first 60 patients have enrolled, the trial will pause temporarily and PET with fluciclovine will be evaluated in these patients. If there is evidence of its ability to predict event-free survival, the trial will continue as planned. However, if PET with fluciclovine is shown to be an ineffective biomarker at this point, subsequent patients who join the trial will receive an MRI scan using spectroscopy as the additional scan.
The primary objective of this trial is to determine whether these biomarkers obtained early in the course of treatment can stratify patients into risk groups based on overall survival. Secondary objectives include determining whether biomarkers can predict if findings on MRI scans later grow larger or shrink. The investigators will also determine whether combinations of biomarkers better predict patient survival than single biomarkers. Finally, the possibility that biomarkers can predict survival and progression in patients who do not show suspicious findings on MRI early in their disease course will be studied.
ECOG-ACRIN encourages sites to open this trial, which has the potential to improve patient care by validating novel imaging approaches that may have improved accuracy, earlier in the course of the disease. By identifying which patients are at high risk of their GBM progressing, care teams may escalate treatment accordingly while sparing those at low risk unnecessary additional therapy.
Learn more about EAF223/GABLE at ecog-acrin.org.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
