
From the Co-Chairs, June 2020
June 10, 2020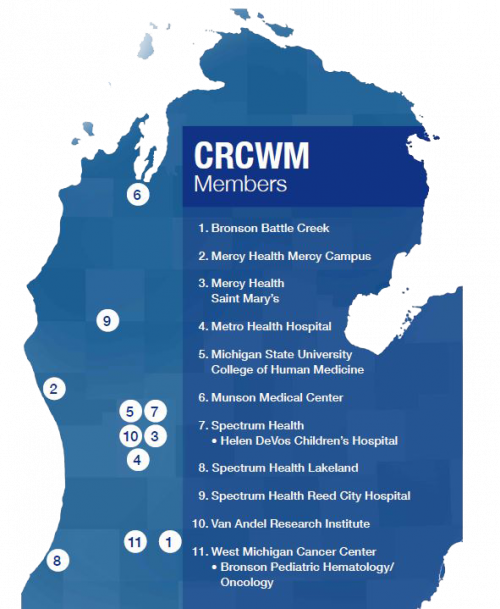
Institution Spotlight: Cancer Research Consortium of Western Michigan
July 29, 2020News in Brief, July 2020

ECOG-ACRIN Research Round-Up
InterAAct/EA2133: Results from this worldwide study, the first randomized trial for inoperable anal cancer, suggest that carboplatin-paclitaxel become the standard treatment for these patients. View the press release or read the article in the Journal of Clinical Oncology.
NCI-MATCH/EAY131 Arm W: Patients with tumors harboring aberrations in the fibroblast growth factor receptor (FGFR) pathway were treated with AZD4547, an oral FGFR1-3 inhibitor, in Arm W of the NCI-MATCH precision medicine trial. Read the article in the Journal of Clinical Oncology.
SOAPP/E2Z02: Data from this study showed that use of higher-than-currently-recommended severity thresholds for symptom alerts for patients receiving outpatient chemotherapy would result in failure to identify and treat many patients requiring clinical intervention for symptoms. Read a summary in The ASCO Post or the full article in JCO Oncology Practice.
NCI-ComboMATCH Laboratory Application Deadline Extended to September 30
The National Cancer Institute (NCI) is currently seeking Clinical Laboratory Improvements Program certified/accredited laboratories that test tumor specimens from patients utilizing next-generation sequencing (NGS) assays to participate in the NCI-ComboMATCH trial. Applications must be submitted by Wednesday, September 30.
NCI-ComboMATCH is a successor to the NCI-MATCH/EAY131 precision medicine trial. Learn more and view the Federal Register notices.
New Resource Available to View NCI-MATCH Trial Publications
ECOG-ACRIN recently created a dedicated web page that lists all publications related to the NCI-MATCH/EAY131 trial. Publications fall into one of two categories: Special Topics About NCI-MATCH or Final Results for Treatment Arms. View the web page now.
Summer 2020 Core Committee Virtual Sessions
This spring marked the first time in ECOG-ACRIN’s history that we could not hold our semi-annual Group Meeting. In an effort to maintain the momentum of our work, we are conducting nine virtual summer core committee sessions, which would have otherwise occurred in-person during the Spring 2020 Group Meeting.
At this time, three of the nine sessions have already taken place, affording committee members the opportunity to discuss new proposals, review active and recently completed trials, and share other important updates.
Fall 2020 Group Meeting Update — As of late July 2020
The ECOG-ACRIN Fall 2020 Group Meeting, scheduled to occur in Fort Lauderdale, FL, from Wednesday, October 21 – Friday, October 23, will not be an in-person event. Due to the evolving and unpredictable nature of the COVID-19 pandemic, we are making preparations to hold the meeting virtually. Please continue to hold these dates on your calendar for the virtual meeting.
A formal announcement is forthcoming shortly – please keep an eye on your email inbox.
TMIST Participant Recruitment Materials Now Available in Chinese and Korean
 These translated materials, which are also available in Spanish, include thank you and appointment reminder cards, a clinic poster and phone script, and templates for letters/emails that sites can customize to send to women scheduled for a mammogram. These and other educational resources for patients and research staff are available for download via the CTSU or the TMIST educational materials page on the ECOG-ACRIN website.
These translated materials, which are also available in Spanish, include thank you and appointment reminder cards, a clinic poster and phone script, and templates for letters/emails that sites can customize to send to women scheduled for a mammogram. These and other educational resources for patients and research staff are available for download via the CTSU or the TMIST educational materials page on the ECOG-ACRIN website.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
