
From the Co-Chairs, January 2021
January 25, 2021
Institution Spotlight: Laura and Isaac Perlmutter Cancer Center at NYU Langone Health
February 25, 2021News in Brief, February 2021

Spring 2021 Group Meeting Will Be Virtual
Save the date for the Virtual Spring 2021 Group Meeting, taking place online from Wednesday, April 28 – Friday, April 30. The format will be similar to that of the Fall 2020 Group Meeting. A more detailed schedule will be released in the coming weeks, and registration is scheduled to open in early March. An email will be sent to all ECOG-ACRIN members when registration is available.
Please note: Virtual Spring 2021 Group Meeting sessions are open to staff from ECOG-ACRIN member institutions, ECOG-ACRIN advocates, NCI/NIH employees, and invited guests only. We are unable to accommodate attendees from non-member institutions or industry. Please email gmp@ecog-acrin.org with any questions.Fourth Month of Record Accrual for TMIST
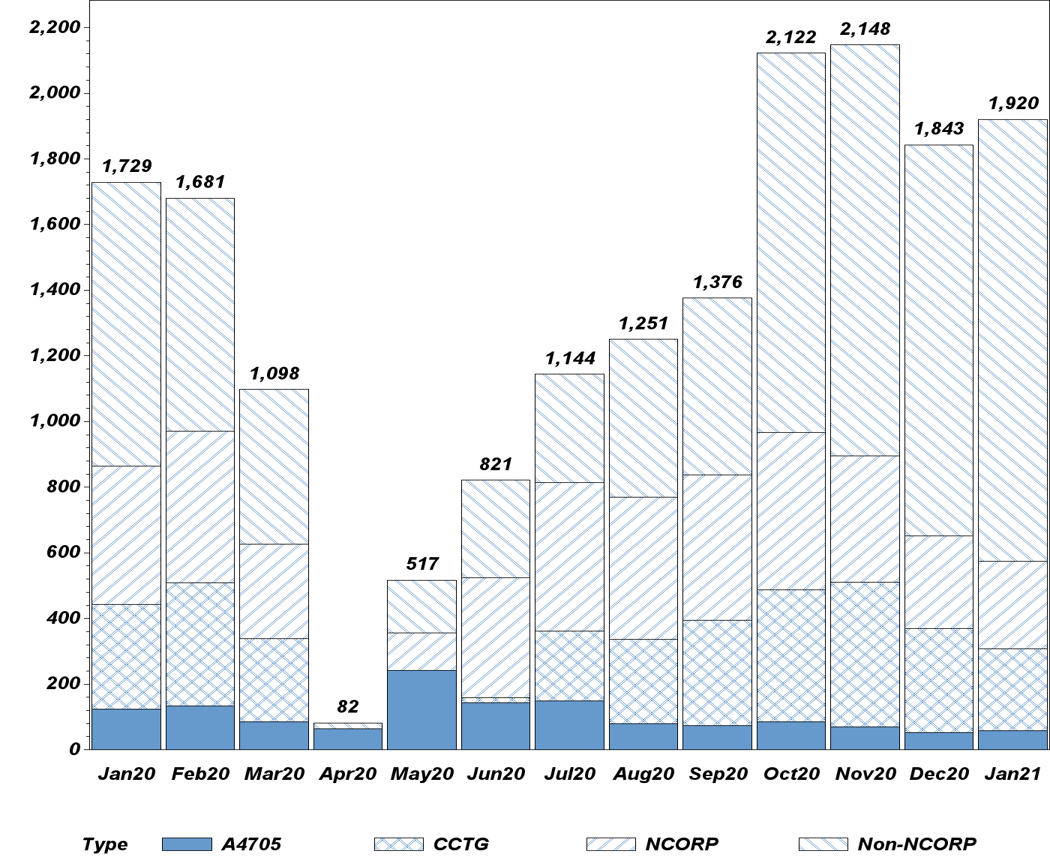
For the fourth month in a row, the TMIST breast cancer screening trial posted strong accrual in January. Total study enrollment stands at 39,779 participants at 106 sites in the US and worldwide (as of February 21). So far, nearly 20% of US participants in TMIST are Black, giving the study the distinction of recruiting one of the most racially diverse populations for any NCI-funded clinical trial.
TMIST continues to recruit new sites! The three most recent sites to open the trial are Mount Sinai Hospital (11028), Carle Physician Group-Mattoon/Charleston (IL393), and Ochsner LSU Health Saint Mary’s Medical Center (LA138). If your site is interested in offering this trial to your constituents, email TMIST@acr.org for a conversation about the study requirements, reimbursement/payment structure and/or to start the application process.
2021 Genitourinary Cancers Symposium: EA Featured Two Trials in Progress
- Bladder Cancer – EA8185 / INSPIRE. This randomized phase II study (n=114) is seeking a new therapeutic approach for patients with Stage 3, node-positive urothelial cancer of the bladder, which tends to behave differently than metastatic disease. Most bladder cancer experts treat node-positive patients with neoadjuvant chemotherapy followed by definitive therapy such as chemoradiation or radical cystectomy and lymph node dissection. However, given the lack of clinical studies, it is hard to tease out the benefit of systemic therapy or a bladder preservation approach in this subset of patients. Furthermore, the advent of immunotherapy has revolutionized the care of bladder cancer patients. INSPIRE is evaluating bladder-sparing chemoradiation with and without durvalumab immunotherapy. Learn more about INSPIRE.
- Prostate Cancer – EA8191 / INDICATE. There is significant variability in the use, nature, and timing of radiation and systemic therapies for patients with post-prostatectomy biochemical recurrence. Furthermore, the available treatments yield high rates of second recurrence. This randomized phase III study (n=480) proposes that PET scan-based treatment intensification will improve progression-free survival relative to non-tailored, standard therapy. It aims to show the clinical significance, if any, of findings seen on PET imaging but not on conventional imaging. The results may help determine if therapeutic intensification strategies based solely on PET findings are safe and warranted. Learn more about INDICATE.
Heather Wakelee, MD Appointed Deputy Director of Stanford Cancer Institute
 Beginning January 2021, Dr. Heather Wakelee assumed the role of deputy director of Stanford Cancer Institute, as well as division chief of oncology in the Department of Medicine. She has been a faculty member at Stanford since 2003 and was promoted to professor in 2017. At Stanford, Dr. Wakelee leads the thoracic malignancies clinical research group and directs the Stanford clinical trials office. She has been an active ECOG-ACRIN member for many years. She currently co-chairs the Thoracic Cancer Committee, is the voting PI for Stanford on the Principal Investigator Committee and is a member of the Executive Committee. Additionally, she received the ECOG-ACRIN Young Investigator Award in 2015.
Beginning January 2021, Dr. Heather Wakelee assumed the role of deputy director of Stanford Cancer Institute, as well as division chief of oncology in the Department of Medicine. She has been a faculty member at Stanford since 2003 and was promoted to professor in 2017. At Stanford, Dr. Wakelee leads the thoracic malignancies clinical research group and directs the Stanford clinical trials office. She has been an active ECOG-ACRIN member for many years. She currently co-chairs the Thoracic Cancer Committee, is the voting PI for Stanford on the Principal Investigator Committee and is a member of the Executive Committee. Additionally, she received the ECOG-ACRIN Young Investigator Award in 2015.
Research Results
- Head and Neck Cancer – Data from a correlative study for E2399 justifies using patient questionnaires as surrogates for objective measures of swallowing disability in large treatment trials of patients with head and neck cancers. The benefits of questionnaires include low cost, ease of administration, and the ability to repeat measures frequently. While objective measures provide a rich source of data, they are costly and require specialized staff. This analysis is the first attempt to validate self-report measures against an objective measure of function (modified barium swallow). Cmelak AJ. Cancers Head Neck. December 22, 2020
- Head and Neck Cancer – A secondary analysis from the ACRIN 6685 trial provides a detailed report of lymph node pathology from FDG-PET/CT findings prospectively collected from patients with cN0 head and neck squamous cell carcinoma. The new report concludes that levels at greatest risk for nodal disease in cN0 in terms of ipsilateral neck dissection are level I (oral cavity), II (pharynx), and VI (larynx). The authors suggest that these data be considered when treating patients presenting with cN0. This is the first study to comprehensively report the incidence, location, and risk of metastases in cN0 in the FDG-PET/CT era. Stack BC. Otolaryngol Head Neck Surg. November 24, 2020
- Lymphoma – Results from E1412, a signal-seeking randomized phase II trial, show that the addition of lenalidomide to R-CHOP improves outcomes in patients with newly diagnosed diffuse large B-cell lymphoma. These results provide the impetus to study lenalidomide and novel lenalidomide analogues. They also highlight the importance of trial design when incorporating biomarkers in frontline studies. Nowakowski GS. J Clin Oncol. February 8, 2021
- NCI-MATCH – The final results of Arm Y show that the oral pan-AKT inhibitor capivasertib had a clinically significant objective response rate of 28.6% in 35 patients with AKT1 E17K-mutated metastatic tumors, including one complete response. At six months, progression-free survival overall was 50%. Capivasertib was discontinued because of adverse events in 11 of 35 patients (31%). Kalinsky KM. JAMA Oncol. December 30, 2020
- Prostate Cancer – Reliance on prostate-specific antigen (PSA) alone is an inadequate strategy to monitor patients undergoing treatment for metastatic hormone-sensitive prostate cancer. Prostate cancer can get worse on scans even with low PSA and/or no or small changes in PSA. A retrospective analysis of all patients in the CHAARTED trial (E3805) examined the concordance of PSA rise and radiographic progression in patients with metastatic hormone-sensitive prostate cancer. The research concludes that imaging should be added to PSA testing to monitor these patients. Bryce AH. Eur Urol Oncol. December 6, 2020
2021 SITC Fellowship Opportunities
The Society for Immunotherapy of Cancer (SITC) is dedicated to assisting investigators in advancing the science of immunotherapy and developing new therapeutic approaches. To further this goal, SITC offers four SITC Fellowships, totaling $450,000 in funding. Additional funding opportunities may be available, so please visit the SITC website for the most up-to-date information. Applications are due by April 2 at 11:59 pm EST.
We encourage you to share these significant funding opportunities with early career scientists in your network to help advance clinical and translational cancer immunotherapy research by displaying the linked poster or sharing the SITC Fellowship opportunities website.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
