
From the Co-Chairs, November 2021
November 16, 2021
ECOG-ACRIN Research at ASH 2021
December 22, 2021News in Brief, December 2021

Janice Mehnert Becomes Melanoma Committee Co-Chair
 Janice Mehnert, MD is the newly appointed co-chair of the ECOG-ACRIN Melanoma Committee, replacing Craig L. Slingluff, MD. Dr. Mehnert is the associate director for clinical research and the director, Melanoma and Cutaneous Medical Oncology at NYU Langone’s Perlmutter Cancer Center. She specializes in both skin cancer and in phase I clinical trials for all types of advanced cancers.
Janice Mehnert, MD is the newly appointed co-chair of the ECOG-ACRIN Melanoma Committee, replacing Craig L. Slingluff, MD. Dr. Mehnert is the associate director for clinical research and the director, Melanoma and Cutaneous Medical Oncology at NYU Langone’s Perlmutter Cancer Center. She specializes in both skin cancer and in phase I clinical trials for all types of advanced cancers.
At Perlmutter, Dr. Mehnert works to expand the availability of clinical trials across locations in Manhattan, in Brooklyn, and on Long Island, bringing cutting-edge studies to people who receive a cancer diagnosis. She is passionate about using precision oncology and immunotherapy to identify effective treatments for advanced cancer. Dr. Mehnert was part of a team whose work led to the US Food and Drug Administration approval of immunotherapy for aggressive neuroendocrine tumors such as small cell lung cancer and Merkel cell carcinoma. For the last decade, she has also led a NCI–funded laboratory focused on developing more precise strategies for the management of melanoma.
Dr. Mehnert has held leadership positions on several international committees, including the Steering Committee of the Society for Melanoma Research and the NCI’s Investigational Drug Steering Committee. She is active with multiple patient advocacy groups including AIM at Melanoma. She is also a past chair of the American Society of Clinical Oncology’s Melanoma and Skin Cancer Scientific Committee. She has authored more than 100 publications, book chapters, and abstracts.
NCI Pancreas Task Force Elects Efrat Dotan as Vice Chair
 In a recent election, NCI Pancreas Task Force members selected Efrat E. Dotan, MD (Fox Chase Cancer Center) as vice chair, replacing E. Gabriela Chiorean, MD (University of Washington/Fred Hutchinson Cancer Research Center). The task force is part of NCI’s Gastrointestinal Cancer Steering Committee.
In a recent election, NCI Pancreas Task Force members selected Efrat E. Dotan, MD (Fox Chase Cancer Center) as vice chair, replacing E. Gabriela Chiorean, MD (University of Washington/Fred Hutchinson Cancer Research Center). The task force is part of NCI’s Gastrointestinal Cancer Steering Committee.
Dr. Dotan chairs the ECOG-ACRIN Geriatric Oncology Working Group, leading the development of clinical trials for older adults that focus on treatments, identification of risk factors, and biomarkers of aging. The now enrolling EA2186/GIANT study for advanced pancreas cancer is one example; others are in development.
Research Results
Breast Cancer—This analysis focuses on the tolerability of bevacizumab and chemotherapy among participants in the phase III randomized E5103 trial. A sustained and cumulative burden of across-the-board toxicities, which were not necessarily all recognized as high-grade adverse events (AEs), contributed to patients discontinuing treatment early. The authors suggest that patients with neuropathic all-grade AEs may require additional attention to prevent deterioration in their physical well-being. Ip EH. Cancer. November 2021
Breast Cancer Survivorship—New data from the TAILORx trial in women with early breast cancer measured fatigue, endocrine symptoms, and quality of life over three years. Adjuvant chemotherapy plus endocrine therapy was associated with greater fatigue and endocrine symptoms at 3 and 6 months than endocrine therapy alone. These differences lessened over time, demonstrating early chemotherapy effects more than long-term ones. Treatment arm differences in endocrine symptoms were more evident in postmenopausal patients. Garcia SF. Cancer. October 2021
Leukemia—Tipifarnib as maintenance therapy did not improve disease-free survival in patients with acute myelogenous leukemia at high risk of relapse, according to the phase III randomized E2902 trial results. Luger SM. Leuk Res. December 2021
NCI-MATCH—Providing cancer patients with education (web-based) about genetics before informing them of their tumor test results increases understanding, according to the COMET (EAQ152) randomized study results. A subgroup of patients with advanced cancer in the NCI-MATCH precision medicine trial was randomized to education versus usual care. Although the intervention did not significantly reduce distress, women had reductions in cancer-specific distress. Refinements to the intervention could benefit low-literacy groups and men. Bradbury AR. Cancer. December 2021
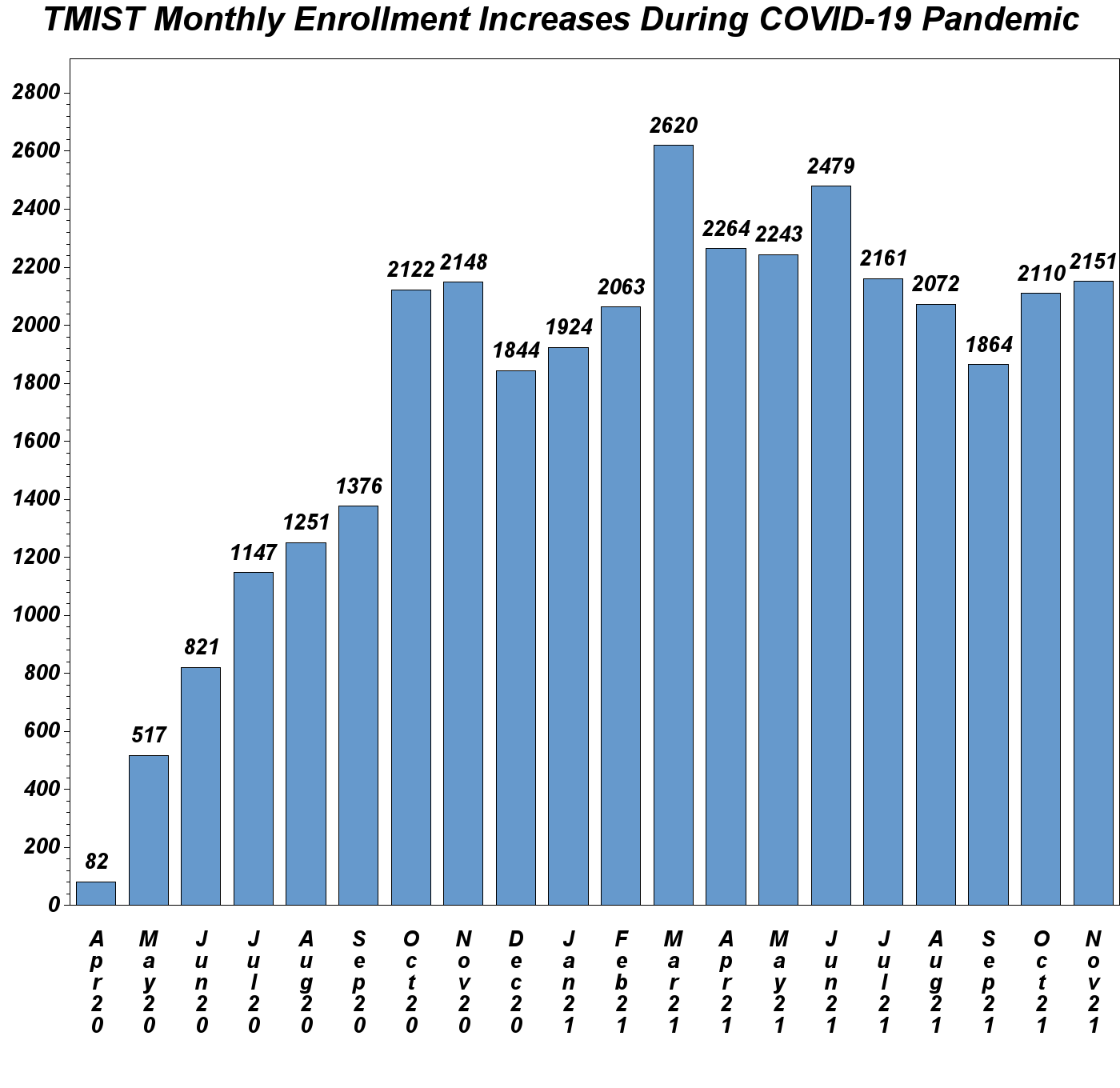
TMIST Enrollment Tops 61,000
The study chair for this trial is Etta Pisano, MD (Beth Israel Deaconess Medical Center, Harvard Medical School, and the American College of Radiology).
The fastest growing National Cancer Institute (NCI)-sponsored trial of the COVID-19 era, enrollment in the Tomosynthesis Mammographic Imaging Screening Trial (TMIST) has nearly doubled in the past 12 months alone. Total accrual in stands at 61,501 participants among 118 participating sites as of December 20, 2021.
“A testament to the fact that the TMIST study question remains unanswered, and that participating sites are effectively communicating with women in their communities, TMIST continues grow,” says Dr. Pisano. “I urge practices with tomosynthesis and digital mammography to visit acr.org/TMIST, read this card, watch this video and contact TMIST staff to find out how and why to take part in TMIST.”
Important updates:
- A CIRB-approved TMIST video is now available here on YouTube
- Education and recruitment materials have been updated and are posted here
- The supplemental trial EAQ201 is now open to assess COVID-19-related financial hardship and distress in women who decline participation in TMIST
- The Love Research Army, part of the Dr. Susan Love Foundation, will soon launch an awareness campaign to promote TMIST participation among its members. Stay tuned for more details in the next newsletter.
Save the Date: Spring 2022 ECOG-ACRIN Group Meeting
Mark your calendar for the Spring 2022 Group Meeting, taking place Wednesday, May 4 - Friday, May 6. ECOG-ACRIN is closely monitoring the status of the COVID-19 pandemic and will make a final decision regarding meeting format closer to the spring. If some or all of the meeting can be held in person, the location will be the Chicago Marriott Downtown Magnificent Mile. We will release additional details in the new year, and expect to open registration in February. Look for an email to all ECOG-ACRIN members when registration is available.
Reminder: Access Resources from Previous Group Meetings
- Meeting Presentations: Registered attendees may visit Attendee Hub to access Fall 2021 session agendas and recordings for on-demand viewing through January 20, 2022. Attendee Hub is available via any web browser or mobile device. Log in with your first name, last name, and the email address you used to register for the meeting.
- Training and Education Materials: We offer several pre-recorded educational presentations, geared toward clinical research staff, to download and view on demand. These resources are available during and after the meeting to anyone with member log-in credentials, regardless of meeting attendance. To access the materials, log in to the Clinical and Administrative Resources section of the website.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
