
From the Co-Chairs, June/July 2021
July 7, 2021
Institution Spotlight: Montefiore Health System and Albert Einstein Cancer Center
August 17, 2021News in Brief, August 2021

NCI-MATCH Trial News and Updates
The study chairs for this trial are Alice Chen, MD (NCI) and Keith Flaherty, MD (ECOG-ACRIN). The study co-chairs are Lyndsay Harris, MD (NCI) and Peter O’Dwyer, MD (ECOG-ACRIN).
- ECOG-ACRIN encourages investigators and research staff to keep NCI-MATCH top-of-mind when considering treatment options for patients, and to share the following articles with local advocate communities: a CURE story on NCI-MATCH and other basket trials, and an ECOG-ACRIN Advocacy Blog feature
- Accrual is complete for Arm M (TAK-228 in patients with TSC1 or TSC2 mutations)
- Accrual is temporarily suspended for the recently activated Arm Z1M (relatlimab and nivolumab in tumors with LAG-3 expression with MMR deficiency). This arm will reopen after the study team processes an amendment with updated safety information.
- The June 2021 NCI-MATCH Site Newsletter is still available on the CTSU website. Topics include the new Z1M arm, unique molecular targets of the open arms, Arm H expansion following promising activity, resources for sites, and an NCI-MATCH highlights video that premiered at the Spring Group Meeting. To view, open the EAY131 protocol page on the CTSU website, navigate to the “Documents” tab and then “Education and Promotion.”
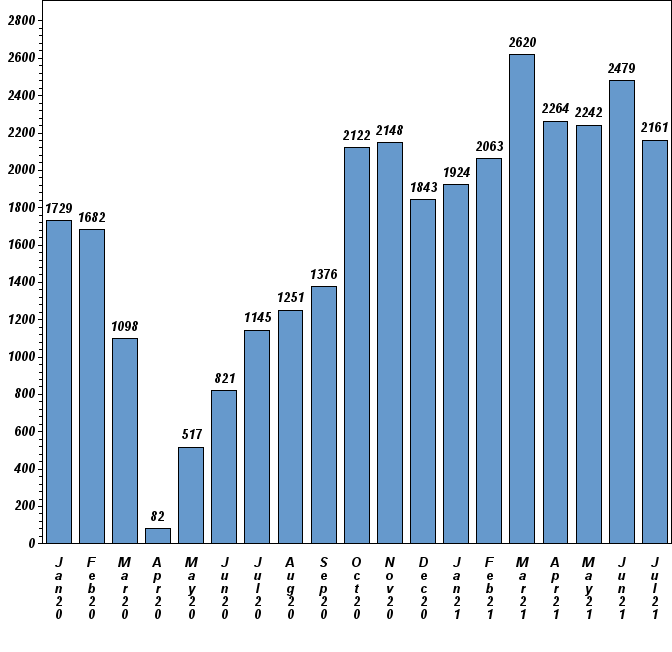
TMIST Enrollment Remains Strong as Leaders Continue to Seek New Sites
The study chair for this trial is Etta Pisano, MD (Beth Israel Deaconess Medical Center, Harvard Medical School, and the American College of Radiology).
Monthly accrual to the TMIST breast cancer screening trial continues at a steady pace (see below). Total enrollment stands at 53,104 women as of August 16. New sites may join this trial! If your site is interested in offering this trial to your constituents, email TMIST@acr.org to discuss the study requirements, reimbursement/payment structure, and how to start the application process.
Submit an Abstract for the Virtual 2021 Young Investigator Symposium
The ECOG-ACRIN Cancer Research Group is accepting research abstracts through September 15 from young investigators interested in presenting their research as part of the upcoming Virtual Fall 2021 Group Meeting. You may be eligible to apply if you are engaged in clinical, translational, imaging, or basic cancer research or in non-cancer-related research that has application to cancer biology, prevention, screening, diagnosis, imaging, or treatment. View full eligibility criteria and the official abstract submission form.
Please note that due to the COVID-19 pandemic the entire Fall 2021 Group Meeting will be virtual. The exact date/time of the Young Investigator Symposium is not yet confirmed, but it will take place in late October, in proximity to the Virtual Group Meeting dates of October 20 – 22. The deadline for submissions is Wednesday, September 15, 2021, 11:59 PM (Eastern).
Andrew Evens To Lead Clinical Innovation and Data Analytics at Rutgers
 Andrew M. Evens, DO, MSc, co-chair of ECOG-ACRIN’s Lymphoma Committee, commences a new role at Rutgers Biomedical and Health Sciences, associate vice chancellor for Clinical Innovation and Data Analytics. In this position, he will lead a multidisciplinary team of analytic experts, epidemiologists, decision scientists, statisticians, translational researchers, hospital and practice administrators, and clinicians. “Via strategic synthesis and analysis of multi-source data, including the integration of imaging and biologic information, we will produce robust decision models to help improve the acute and long-term health of our patients in a more precise and individualized manner,” says Dr. Evens. Read the full press release.
Andrew M. Evens, DO, MSc, co-chair of ECOG-ACRIN’s Lymphoma Committee, commences a new role at Rutgers Biomedical and Health Sciences, associate vice chancellor for Clinical Innovation and Data Analytics. In this position, he will lead a multidisciplinary team of analytic experts, epidemiologists, decision scientists, statisticians, translational researchers, hospital and practice administrators, and clinicians. “Via strategic synthesis and analysis of multi-source data, including the integration of imaging and biologic information, we will produce robust decision models to help improve the acute and long-term health of our patients in a more precise and individualized manner,” says Dr. Evens. Read the full press release.
Heather Wakelee Honored by GO2 Foundation for Lung Cancer
 Heather A. Wakelee, MD, co-chair of ECOG-ACRIN’s Thoracic Cancer Committee, voting member for Stanford University on the Principal Investigator Committee, member of the Executive Committee, member of the Nominating Committee, and the 2015 ECOG-ACRIN Young Investigator of the Year, is the recipient of the GO2 Foundation for Lung Cancer’s 2021 Bonnie J. Addario Lectureship Award. “Given women are disproportionately impacted by lung cancer, the advancements in lung cancer research by women like Dr. Wakelee are incredibly meaningful to the lung cancer community,” says Bonnie J. Addario, foundation co-founder and chair. Read the full press release.
Heather A. Wakelee, MD, co-chair of ECOG-ACRIN’s Thoracic Cancer Committee, voting member for Stanford University on the Principal Investigator Committee, member of the Executive Committee, member of the Nominating Committee, and the 2015 ECOG-ACRIN Young Investigator of the Year, is the recipient of the GO2 Foundation for Lung Cancer’s 2021 Bonnie J. Addario Lectureship Award. “Given women are disproportionately impacted by lung cancer, the advancements in lung cancer research by women like Dr. Wakelee are incredibly meaningful to the lung cancer community,” says Bonnie J. Addario, foundation co-founder and chair. Read the full press release.
E2112 Final Results Appear in the Journal of Clinical Oncology
E2112 was a double-blind, placebo-controlled phase III trial led by Roisin M. Connolly, MB, BCh, MD (University College Cork, Ireland), that investigated whether the addition of the histone deacetylase inhibitor entinostat to exemestane would improve progression-free and/or overall survival in patients with advanced hormone receptor–positive, human epidermal growth factor receptor 2–negative breast cancer resistant to a nonsteroidal aromatase inhibitor. Despite supporting preclinical and phase II clinical data, the trial did not meet either co-primary endpoint and thus does not support a role for entinostat in this setting. Pharmacodynamic analysis confirmed target inhibition in entinostat-treated patients. Read the original manuscript (subscription required).
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
