
News in Brief, July 2020
July 29, 2020
EA Launches Anti-Racist Agenda with Health Equity Inaugural Meeting
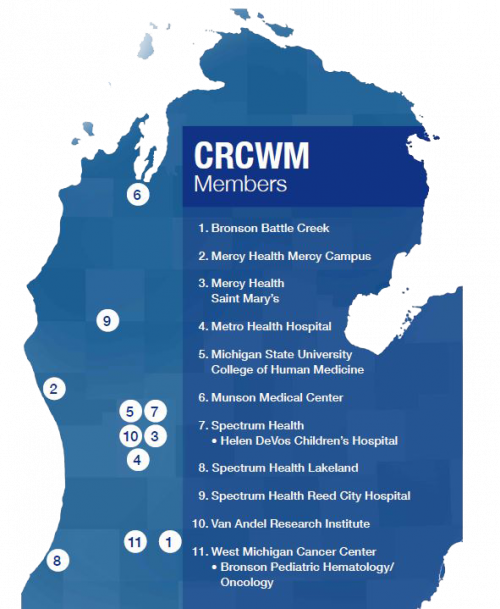
July 29, 2020Institution Spotlight: Cancer Research Consortium of Western Michigan



By Kathleen J. Yost, MD
ECOG-ACRIN Principal
Investigator Committee
The Cancer Research Consortium of West Michigan (CRCWM or the Consortium) was formed in August 2014 when the Grand Rapids Clinical Oncology Program (GRCOP) and the Kalamazoo Community Clinical Oncology Program joined together, with the support of an NCI Community Oncology Research Program (NCORP) grant. Both programs had previously been legacy participants in the NCI Community Clinical Oncology Program, offering national clinical trials through a community-based clinical research program.
 Today, CRCWM offers local access to and data management support for over 150 cancer research studies across its nine member hospitals and health care systems (currently 11 sites). The Consortium works to provide the highest quality care for cancer prevention and treatment. CRCWM continues to provide access to the latest cancer research both nationally and locally.
Today, CRCWM offers local access to and data management support for over 150 cancer research studies across its nine member hospitals and health care systems (currently 11 sites). The Consortium works to provide the highest quality care for cancer prevention and treatment. CRCWM continues to provide access to the latest cancer research both nationally and locally.
CRCWM (then GRCOP) became an ECOG-ACRIN (EA) member in 1996. Its director, Connie Szczepanek, has served in a leadership role for the Consortium since 1998. It has had four principal investigators over this time who serve or have served on the EA Principal Investigator Committee. My colleague Dr. Sreenivasa Chandana is a strong proponent of ECOG-ACRIN research and a steady enroller in its trials. Joan Westendorp, one of our nurse leaders and current CRCWM Consortium Governance Board Chairperson, serves on multiple ECOG-ACRIN committees including: Head and Neck Cancer, Cancer Control and Survivorship, and Cancer Care Delivery Research. She is also an auditor for EA. Barb Lomasney recently became an ECOG-ACRIN auditor, too.
Like all sites right now, CRCWM is working hard to ensure clinical trials continue despite the challenges posed by the pandemic. Enrollment to the TMIST (EA1151) breast cancer screening trial stalled when the country shut down in March, so now we are focused on finding creative ways to restore momentum. Dr. Jon Notarnicola and Dr. Christina Jacobs have been vital to TMIST recruitment efforts, collaborating with primary care practices and developing resources to highlight the importance of the study. We also hold regular meetings to identify issues and accrual barriers – as well as solutions – for TMIST.
As an NCORP site, CRCWM has a special interest in ECOG-ACRIN’s Cancer Care Delivery Research and Cancer Control and Survivorship studies. We participated in the pilot testing for EAQ161CD, Biomarker Testing in Common Solid Cancers: An Assessment of Current Practices in Precision Oncology in the Community Setting. We have also found that studies concerning financial toxicity, like EAQ162CD, resonate deeply with the communities we serve. Our site in Kalamazoo accrued over 60 participants to EA1141, which found that abbreviated MRI outperforms 3-D mammography at finding cancer in dense breasts. Newer EA trials we are prioritizing include EAZ171, studying neuropathy in African American women, and EA2185, comparing two methods of monitoring patients with pancreatic cysts.
CRCWM is particularly proud of its advocate involvement and has its own Patient Advocate Committee, formed in 2008. The group, co-chaired by Sue Schroder and Marty Smith, brings a unique perspective to CRCWM’s research efforts. The Committee also played a key role in developing patient-friendly content for the CRCWM website. Together with the investigators, research staff, and patients, the advocates help make CRCWM the exceptional research program that it is.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
