
News in Brief, February 2024
February 9, 2024
Trial Spotlight: Max Kates on EA8212/BRIDGE for Patients with Non-Muscle Invasive Bladder Cancer
February 9, 2024Institution Spotlight: Baptist Memorial Health Care/Mid-South Minority Underserved NCORP
By Baptist NCORP Staff

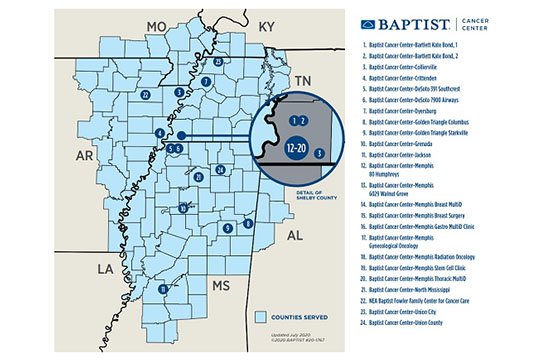
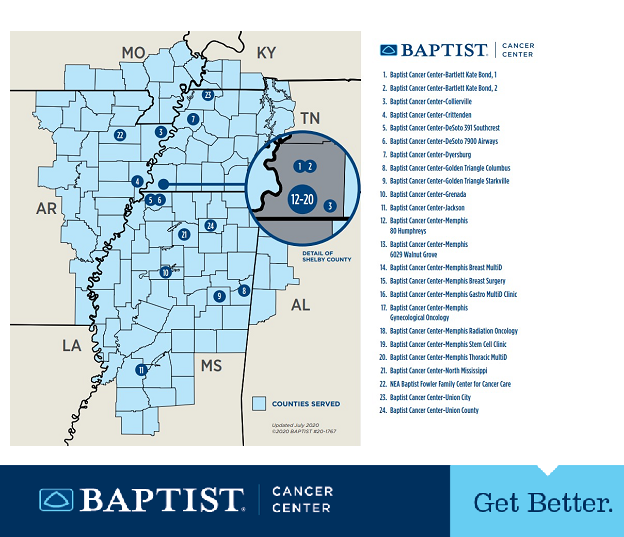
Since its creation in 2014, the Baptist Memorial Health Care/Mid-South Minority Underserved NCORP (Baptist NCORP) has created the infrastructure for oncology clinical research across the Baptist Memorial Health Care Corporation’s (BMHCC) facilities in western Tennessee, northern and central Mississippi, and eastern Arkansas. However, the service area population resides in 111 counties across six states in the mid-south region of the United States, including counties in southwestern Kentucky, southeastern Missouri, and northwestern Alabama. Demographically, 44% are Delta Regional Authority counties, which are congressionally identified as the most socioeconomically challenged in the country.
Headquartered in Memphis, Tennessee, BMHCC, with its 21 hospitals, is the largest healthcare organization in the southeastern United States. We serve a racially, socioeconomically, and geographically diverse patient population, ranging from severely underserved rural residents of the Mississippi Delta, to inner city metropolitan areas in Memphis and Jackson, Mississippi, to wealthy suburban metropolitan areas. We strive to bring high-quality cancer care and access to cutting-edge research to all patients across our network.
Over the course of its grant funding, the Baptist NCORP has successfully enrolled patients into many cooperative group clinical trials across the demographic spectrum, including suburban, urban, and rural patient populations. In this past year, we were awarded “Gold” status by the National Cancer Institute (NCI) for patient enrollment and have been commended for our success in enrolling patients from underrepresented segments of the oncology population. Approximately 35% of patients enrolled to our clinical trials are African American, which largely mirrors the demographics of the overall population that we serve across our hospital system.
We are especially dedicated to conducting clinical research in the state of Mississippi, where there is no NCI-designated cancer center. We have active investigators throughout the state in several oncology specialties and are able to accrue patients to treatment-related studies, cancer prevention, symptom control, and supportive care studies, as well as cancer care delivery research studies.
We opened the state’s only site accruing to the EA1151 study, Tomosynthesis Mammographic Imaging Screening Trial (TMIST), at our Mississippi Baptist Hospital-Desoto. To date, we have accrued over 280 patients combined at our site in Mississippi and our site in Memphis.
We have been an active ECOG-ACRIN research base member since our grant funding began in 2014 and have over 350 enrollments credited to ECOG-ACRIN in the last 5 years. Below, we highlight our leadership and team.
 Philip Lammers, MD, MSCI, is the institutional ECOG-ACRIN principal investigator (PI) and has served as the Medical Director of Oncology Research at Baptist since joining Baptist in 2018. He has been the site PI of over 20 ECOG-ACRIN studies throughout his career, is an active member of the ECOG-ACRIN Community Cancer Committee, and a past member of the ECOG-ACRIN Health Equity Committee.
Philip Lammers, MD, MSCI, is the institutional ECOG-ACRIN principal investigator (PI) and has served as the Medical Director of Oncology Research at Baptist since joining Baptist in 2018. He has been the site PI of over 20 ECOG-ACRIN studies throughout his career, is an active member of the ECOG-ACRIN Community Cancer Committee, and a past member of the ECOG-ACRIN Health Equity Committee.
 Raymond Osarogiagbon, MBBS, FACP, is our NCORP PI and a world leader in lung cancer research. Under his leadership, our lung research program consistently enrolls well for a variety of studies. Our Baptist Memphis site is currently the leading national accruing site to the EA5162 clinical trial, Randomized Phase III Study of Combination Osimertinib and Bevacizumab Versus Osimertinib Alone as First-Line Treatment for Patients with Metastatic EGFR-Mutant Non-Small Cell Lung Cancer, and we were the top minority enrolling site to the EAQ171CD study, Implementing a Virtual Tobacco Treatment in Community Oncology Practices: Smoke Free Support Study 2.0.
Raymond Osarogiagbon, MBBS, FACP, is our NCORP PI and a world leader in lung cancer research. Under his leadership, our lung research program consistently enrolls well for a variety of studies. Our Baptist Memphis site is currently the leading national accruing site to the EA5162 clinical trial, Randomized Phase III Study of Combination Osimertinib and Bevacizumab Versus Osimertinib Alone as First-Line Treatment for Patients with Metastatic EGFR-Mutant Non-Small Cell Lung Cancer, and we were the top minority enrolling site to the EAQ171CD study, Implementing a Virtual Tobacco Treatment in Community Oncology Practices: Smoke Free Support Study 2.0.
 Our Cancer Care Delivery Research (CCDR) and Cancer Prevention Team is an integral part of our NCORP work and is led by Alyssa Throckmorton, MD, a breast surgeon, and a member of the Alliance cooperative group board of directors. We strive to have at least three CCDR studies open to accrual at all times across our sites. In addition to being a leader in accruals to the EAQ171CD study, given the large number of accruals throughout the Baptist NCORP, Dr. Throckmorton will be an author on the upcoming manuscript for the E1Q11 study, EROS: Engendering Reproductive Health within Oncologic Survivorship. To complement our CCDR activity in ECOG-ACRIN, we are active in CCDR studies across other research bases; we were the third highest accruing site to the recently completed Wake Forest study, Assessing Effectiveness and Implementation of an EHR Tool to Assess Heart Health Among Survivors.
Our Cancer Care Delivery Research (CCDR) and Cancer Prevention Team is an integral part of our NCORP work and is led by Alyssa Throckmorton, MD, a breast surgeon, and a member of the Alliance cooperative group board of directors. We strive to have at least three CCDR studies open to accrual at all times across our sites. In addition to being a leader in accruals to the EAQ171CD study, given the large number of accruals throughout the Baptist NCORP, Dr. Throckmorton will be an author on the upcoming manuscript for the E1Q11 study, EROS: Engendering Reproductive Health within Oncologic Survivorship. To complement our CCDR activity in ECOG-ACRIN, we are active in CCDR studies across other research bases; we were the third highest accruing site to the recently completed Wake Forest study, Assessing Effectiveness and Implementation of an EHR Tool to Assess Heart Health Among Survivors.
 Our malignant hematology research group is led by Salil Goorha, MD. The Baptist NCORP is in the top 10 nationally in total enrollments and is a leader in minority patient enrollments to the National Myelodysplastic Syndromes Study (NHLBI-MDS). As with other disease groups, we are able to enroll patients to lymphoma/leukemia studies across our catchment area.
Our malignant hematology research group is led by Salil Goorha, MD. The Baptist NCORP is in the top 10 nationally in total enrollments and is a leader in minority patient enrollments to the National Myelodysplastic Syndromes Study (NHLBI-MDS). As with other disease groups, we are able to enroll patients to lymphoma/leukemia studies across our catchment area.

Tracy Stewart, Julie Ryder
Throughout the Baptist NCORP, we pride ourselves on team-based care and have engaged research coordinators, data managers, and other research staff across our large network. Our oncology research administration team is currently led by the Assistant Director of Research, Tracy Stewart, MBA, BS, RN, CCRC, along with our Mississippi Research Manager, KiOsha Carlyle, MSN, RN, CCRP, and our Tennessee/Arkansas Research Manager, Julie Ryder, MA, BSN, RN, CCRP.
The Baptist NCORP has been a successful ECOG-ACRIN member for 10 years and we look forward to continuing to work with ECOG-ACRIN in the years to come. We anticipate continuing to grow our presence across our core network, with particular emphasis on expanding our footprint in the state of Mississippi.
We are dedicated to bringing vital ECOG-ACRIN clinical trials to our diverse rural and urban patients in the communities where they live. We believe that the best treatment is a clinical trial and will continue to strive to offer all of our patients access to clinical research no matter whether they live in the Mississippi Delta, the inner city of Memphis, or in suburban areas.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
