
Trial Spotlight: Curtis Pettaway on EA8134/InPACT, the International Study for Patients with Advanced Penile Cancer
June 30, 2023
Arlene Forastiere Honored with ECOG-ACRIN’s Inaugural Remarkable Mentor to Women in Oncology Award
June 30, 2023Game, Set, (NCI-)MATCH
During the General Session of the Spring 2023 Group Meeting, ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) leaders and attendees celebrated the conclusion of accrual to the landmark NCI-MATCH study (EAY131), one of the first and largest precision medicine cancer clinical trials. ECOG-ACRIN launched NCI-MATCH in August 2015 with 10 arms, and the trial grew to 38 treatment arms. Patient enrollment opportunities expanded as new arms opened, with the final two closing in December 2022. Ultimately, 1201 patients received treatment in NCI-MATCH at nearly 1100 US clinical sites.
NCI-MATCH represented an innovative collaboration between investigators at ECOG-ACRIN, the National Cancer Institute (NCI), Alliance for Clinical Trials in Oncology, NRG Oncology, and the SWOG Cancer Research Network, with sponsorship from the NCI through its National Clinical Trials Network (NCTN).
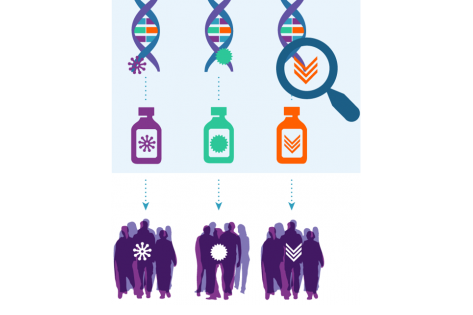
Among its achievements, NCI-MATCH demonstrated that targeting genetic changes in a tumor may be an effective way to treat cancer—and that people with advanced cancer may benefit from tumor testing to help plan their treatment. After all, NCI-MATCH was a signal-finding series of phase 2 trials—and several studies met their primary endpoint. Among the initial 27 arms with outcomes data thus far, 25.9% (7/27) were positive. In addition, each substudy contributes valuable information, with responses observed in arms that did not meet their primary endpoint. Researchers are developing manuscripts for the rest of the remaining treatment arms.
Unexpectedly, in one instance, data from the trial contributed to US Food and Drug Administration (FDA) accelerated approval of a new treatment. Though FDA approval was not the primary objective for NCI-MATCH, patients with any type of solid tumor harboring a BRAF V600E mutation stand to benefit (Salama AKS. J Clin Oncol. August 2020).
“The approval of dabrafenib and trametinib for patients with BRAF V600E mutations was critical because this gene abnormality occurs across many types of rare cancers,” said Peter J. O’Dwyer, MD, ECOG-ACRIN group co-chair and one of the lead investigators of NCI-MATCH. “Patients needed access to this treatment option.”
The trial proved transformative in other ways as well. NCI-MATCH pioneered a new era in cancer medicine—one in which physicians routinely utilize genomic sequencing to help guide their patients’ treatment.
“NCI-MATCH required us to set up the infrastructure that allows precision medicine to drive the first line of evaluation for best therapies for a patient,” said Kathleen N. Moore, MD, site principal investigator (PI) at the University of Oklahoma Stephenson Cancer Center, one of the trial’s highest enrolling centers.
The impact was similar in the community setting: “NCI-MATCH was the first study of this kind that we opened, where we harnessed genomic data to identify potential clinical trial options for our patients,” said Amelia B. Zelnak, MD, site PI at the Georgia NCI Community Oncology Research Program (NCORP), another high-enrolling site. “Now, genomic sequencing is routine in the clinic.”
Another accomplishment of NCI-MATCH was the broad inclusion of patients with diagnoses of uncommon or rare cancers. For example, 38% of the accrual was in a disease other than breast, colon, non-small cell lung, or prostate cancer. Many of these patients had limited or no treatment choices available.
NCI-MATCH played a major role in establishing the value of next-generation sequencing for treatment selection, which will benefit many patients in the future. The data from the trial strongly support tumor testing for all patients with solid tumors.
With so much data, there is much more to come from the NCI-MATCH correlative studies program—stay tuned.
Beyond NCI-MATCH
Lessons learned from NCI-MATCH have already been applied to the next generation of precision medicine trials. ECOG-ACRIN and the NCI are leading the recently launched ComboMATCH precision medicine initiative, testing therapy with new drug combinations guided by tumor biology. ImmunoMATCH’s (iMATCH) first pilot study is underway, investigating how the immune status of a tumor affects the response to targeted treatments with immunotherapy.
Additionally, MyeloMATCH will test treatments based on genetic changes in the cancer cells of people with acute myeloid leukemia or myelodysplastic syndromes. SWOG Cancer Research Network and the NCI are leading both iMATCH and MyeloMATCH. All three precision medicine initiatives are the result of collaboration between the NCI and all five NCTN Groups.
Watch the video below to learn more about the impact of NCI-MATCH.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
