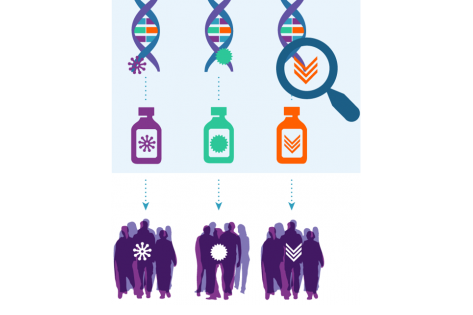
Trial Results: Copanlisib in Patients with PIK3CA-Mutated Tumors
February 24, 2022
Edith Mitchell Reflects on Equity Initiatives at ECOG-ACRIN
February 24, 2022ECOG-ACRIN Sarcoma Working Group Builds Steam in Second Year

 Within the last couple of years, the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) reinforced its commitment to improving outcomes for patients with a particularly rare type of cancer—sarcoma. ECOG-ACRIN added a formal Sarcoma Working Group to the Therapeutic Studies Program. The Group Co-Chairs appointed Kenneth Cardona, MD, FACS (pictured) of the Winship Cancer Institute at Emory University as chair. In addition, they named Richard Riedel, MD (Duke University/Duke Cancer Institute) co-chair, and Ronnie Sebro, MD, PhD (Mayo Clinic in Florida) imaging chair. Together, this team of sarcoma experts is working to launch innovative sarcoma clinical trials through ECOG-ACRIN.
Within the last couple of years, the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) reinforced its commitment to improving outcomes for patients with a particularly rare type of cancer—sarcoma. ECOG-ACRIN added a formal Sarcoma Working Group to the Therapeutic Studies Program. The Group Co-Chairs appointed Kenneth Cardona, MD, FACS (pictured) of the Winship Cancer Institute at Emory University as chair. In addition, they named Richard Riedel, MD (Duke University/Duke Cancer Institute) co-chair, and Ronnie Sebro, MD, PhD (Mayo Clinic in Florida) imaging chair. Together, this team of sarcoma experts is working to launch innovative sarcoma clinical trials through ECOG-ACRIN.
Below, Dr. Cardona (pictured) discusses the Sarcoma Working Group’s progress to date and outlines future plans.
What is the background behind the Sarcoma Working Group, and how did it form?
The Sarcoma Working Group was created just under two years ago. In the 1990s and 2000s, sarcoma research in ECOG-ACRIN was integrated within another disease-focused committee, but the interest slowly faded away. Within the last five to ten years, however, we have seen significant growth and reinvigoration in the investigation of sarcoma—not only at ECOG-ACRIN but nationally and internationally. In recent years, ECOG-ACRIN conducted sarcoma research through its Surgery Committee. That led to my effort, with the support of ECOG-ACRIN leadership, to establish a formal Sarcoma Working Group.
What are your goals for the working group?
Right now, we have three primary goals. First, we want to foster and re-establish an environment of collaboration amongst sarcoma clinicians and centers within the ECOG-ACRIN cooperative group. Secondly, we look to develop histology-specific and potentially tumor-agnostic clinical trials for patients with sarcoma. In addition, we want to conduct surveillance studies for patients with sarcoma. Patients with localized disease are still at high risk of recurrence, but current NCCN Guidelines® are not clear regarding the optimal surveillance strategy. These are the issues we want to investigat, to hopefully make a meaningful impact on patient care.
What initiatives or studies are currently underway?
We have a handful of clinical trials in development within the working group as well as under review with the ECOG-ACRIN Executive Review Committee. In addition, I just recently presented our proposed phase III surveillance study for patients with high risk soft tissue sarcoma to the National Cancer Institute’s (NCI) Division of Cancer Prevention.
We are also working to open the STRASS2 trial here in the US, which would be a huge milestone. STRASS2 (NCT04031677) is studying surgery with or without neoadjuvant chemotherapy treatment for patients with high-risk retroperitoneal sarcoma. It opened in Europe in 2020 through the European Organisation for Research and Treatment of Cancer (EORTC). Opening this trial in the US would be a huge step forward for not only our sarcoma patients, but also for the sarcoma community as we look to create new models of collaboration to conduct studies in a rare disease such as sarcoma.
Years ago, the NCI and EORTC collaborated more regularly to open transatlantic clinical trials, but that became difficult over the years and became more of the exception than the norm. As we began to build the ECOG-ACRIN Sarcoma Working Group, the NCI and EORTC were having parallel discussions on ways to reopen transatlantic trials. STRASS2 emerged as a great opportunity to resume collaboration. If we’re successful, the study could reopen the door to the NCI and EORTC partnering on studies for other cancers, especially rare cancers.
Who currently serves on the working group, and who can participate?
We have around 25 individuals from nearly as many ECOG-ACRIN member sites across the US. Our group is diverse and spans the oncology disciplines: medical oncology, radiation oncology, surgical oncology, orthopedic oncology, and radiology. We are open to investigators of any clinical discipline in the field of oncology, from both academic and community-based sites. We welcome young clinicians, irrespective of a specialty in sarcoma, but also researchers at any point in their careers. We have great senior mentors in our group, yet welcome well-established sarcoma clinicians to help guide and serve as sounding boards for future sarcoma investigators.
How can people from ECOG-ACRIN member sites join the working group?
They can email me and Kelly Galarza, the sarcoma protocol associate at ECOG-ACRIN. We meet monthly via Zoom to discuss concepts and foster collaboration with our sarcoma colleagues from the other NCI cooperative groups: Alliance, SWOG, Children’s Oncology Group, and NRG. Sarcoma is such a rare disease that we cannot afford to have duplicate studies or competing trials, thus we must collaborate and support all efforts being made across the various cooperative groups. This is also the beauty of the sarcoma community—we all know each other and are a welcoming group.
What is sarcoma, and what are some of the clinical trials currently in progress? Learn more at these two advocacy organizations that partner with the ECOG-ACRIN Cancer Research Group:
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
