
Trial Spotlight: Efrat Dotan Gives an Update on the EA2186/GIANT Study for Older Adults with Pancreatic Cancer
August 25, 2023
Now Enrolling: EA7211/STRASS 2 for Patients with High-Risk Retroperitoneal Sarcoma
August 25, 2023Amended Trial: Eligibility Expands in the EA8191/INDICATE Trial for Patients with Prostate Cancer
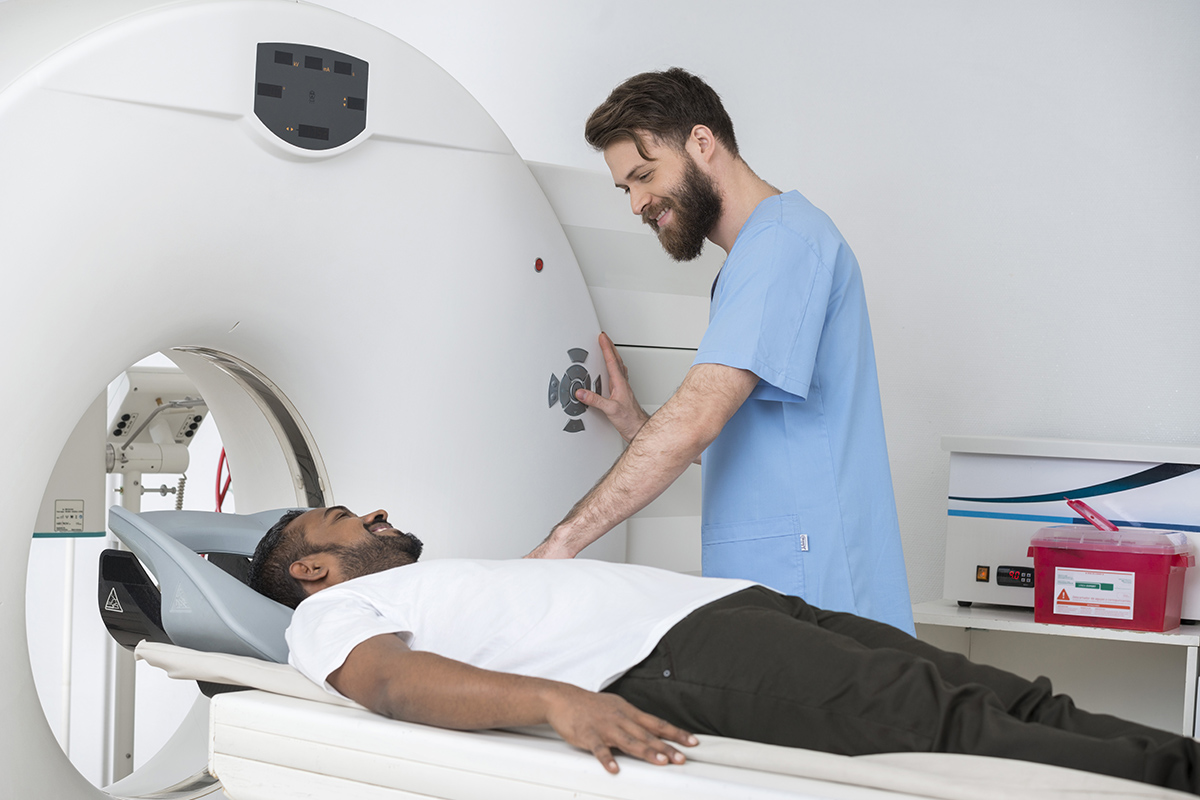
EA8191 – Phase III Study of Local or Systemic Therapy INtensification DIrected by PET in Prostate CAncer Patients with Post-ProstaTEctomy Biochemical Recurrence (INDICATE)
Neha Vapiwala, MD (University of Pennsylvania/Abramson Cancer Center) is the study chair for this trial. The study co-chairs for imaging, medical oncology, and quality of life are Steve Cho, MD (University of Wisconsin/Carbone Cancer Center), Christos Kyriakopoulos, MD (University of Wisconsin/Carbone Cancer Center), and Alicia Morgans, MD (Dana-Farber/Harvard Cancer Center), respectively.
Up to 40% of patients who have had a radical prostatectomy (RP) for their cancer develop biochemical recurrence (BCR) within 10 years of surgery. When this happens, the standard-of-care treatment is radiation therapy (RT) to the prostate bed and pelvic nodes, plus short-term androgen deprivation therapy (STAD). However, even after this salvage therapy, some patients experience a second biochemical recurrence, raising questions about which subgroups of patients may have micrometastatic disease and which patients may benefit from treatment intensification. Improved imaging capabilities that can detect the site(s) of recurrence may help answer these questions and inform treatment guidelines.
The phase 3 EA8191/INDICATE study is using PET imaging to guide treatment for patients with post-prostatectomy biochemical recurrence. The study has two primary objectives. First, for patients without PET evidence of extra-pelvic metastases (defined as outside of standard salvage RT fields), the study will evaluate if the addition of enhanced systemic therapy (apalutamide) to standard-of-care STAD may improve progression-free survival (PFS). Second, for patients with PET evidence of extra-pelvic metastases, the study will evaluate whether the addition of metastasis-directed RT to apalutamide and standard-of-care STAD may prolong PFS.
Androgen hormones can contribute to the growth of prostate cancer cells. Apalutamide is used to help fight prostate cancer by blocking the use of androgens by the tumor cells.
Amendments #7 and #8 were recently activated to expand eligibility and increase PET scanner flexibility including allowable radiotracers. "We are taking necessary steps to keep this study clinically relevant," says Dr. Vapiwala. "As clinical practice evolves and as guidelines adapt, we want EA8191 to evolve and adapt as well. Although difficult with large randomized trials, we are striving to ensure the study remains as patient- and investigator-friendly as possible while staying true to the science."
Please see below for the key changes. The EA8191 Site Newsletter (available on the CTSU) also features a summary of the recent amendments. Please refer to the protocol and change memo for full details (also on the CTSU).
- Eligibility modifications including:
- Minimum prostate-specific antigen (PSA) thresholds based on time to first detectable (any non-zero) PSA after prostatectomy
- Waiver of minimum PSA threshold requirement if baseline PET (PET1) is positive
- The scanner qualification requirement (PET Body) was removed; patients will receive a standard-of-care PET/CT or PET/MR, utilizing any FDA-approved radiotracer for prostate cancer
- For sites not routinely using conventional imaging modalities (CIM) and only using PET for staging:
- PET with an approved radiotracer should be done within 16 weeks prior to Step 0 (registration)
- If PET is negative for extra-pelvic lesions, then baseline CIM is not required
- If PET is positive for extra-pelvic lesions, then the patient should have a baseline CT or MRI for soft tissue lesions and/or a bone scan for osseous lesions
- A short course of anti-androgen therapy such as bicalutamide—given after baseline study PET but prior to study registration—is permitted
- Apalutamide can start up to 21 days after Step 1 (randomization)
- PET imaging may now be utilized as a follow-up modality; it is strongly encouraged to use the same imaging modality for follow-up as was used for baseline eligibility imaging
If you have any questions or are interested in opening the study at your center, please email Diana Ewen, the principal project manager for diagnostic imaging clinical trials at the American College of Radiology.
Learn more about EA8191 at ecog-acrin.org.
The NCTN group study champions are Rana McKay, MD (University of California San Diego/Moores Cancer Center) for Alliance, Bridget Koontz, MD (East Carolina University) for NRG Oncology, and Evan Yu, MD (University of Washington/Fred Hutchinson Cancer Research Center) for SWOG.
This post was updated on August 28, 2023.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
