Now Enrolling: EAQ191 / CARISMA for management of hypertension in patients with metastatic renal cell or thyroid cancer on anti-angiogenic tyrosine kinase inhibitors
October 19, 2020
Trial Results: Genomic study of 6000 NCI-MATCH cancer patients leads to new clinical trial benchmarks
October 19, 2020TMIST Breast Cancer Screening Trial Rebounds from COVID-19 Shutdown of Cancer Screenings
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) has recruited more than 31,000 women at mammography clinics around the world to date. TMIST has enrolled more patients than all other National Cancer Institute (NCI) clinical trials combined since July 1, following the spring-summer, CDC-recommended cancer screening shutdown. October accrual is on pace to be the highest month on record. Already, 101 sites are actively participating in TMIST. At least 50 more have committed to take part and are at various stages of onboarding. Talks continue with several other prospective sites—both international and domestic. As these sites enter the study in the coming months, patient accrual will continue to accelerate.
Below, Etta D. Pisano, MD, principal investigator for TMIST, and Mitchell D. Schnall, MD, PhD, Chairman, Department of Radiology, Penn Medicine, and ECOG-ACRIN Group Co-Chair, provide an update and speak about the continuing relevance of TMIST.
Some radiologists tend to already view 3D mammography as superior to 2D mammography. Has this fully been established as a fact? Are there any misconceptions about this line of thinking?
 Dr. Pisano: “The primary goal of TMIST has often been misportrayed. It is not to determine whether digital breast tomosynthesis finds more cancers than standard 2-D digital mammography, but whether the newer technology may help reduce breast cancer deaths by finding more of the types of advanced cancers likely to kill women.
Dr. Pisano: “The primary goal of TMIST has often been misportrayed. It is not to determine whether digital breast tomosynthesis finds more cancers than standard 2-D digital mammography, but whether the newer technology may help reduce breast cancer deaths by finding more of the types of advanced cancers likely to kill women.
Several factors set TMIST apart from earlier, technology-focused studies. TMIST will develop the world's largest biorepository for women undergoing cancer screening and study the genetic basis of the development of breast cancer. Another important output of the trial may be more scientifically-based recommendations for personalized screening strategies, which is likely why the study has appealed to women who are not ideally served by current age-based screening strategies.”
What is the scientific relevance of TMIST?
 Dr. Schnall: “One size should not fit all in breast cancer screening, and the TMIST trial can show the way to a precision approach to screening. Look, we all know that 3D sees more lesions, but consequently, more women are having additional procedures, including biopsies and excisions. The question that TMIST asks is whether those huge expenditures of time, money, and psychological stress are worth it in terms of better outcomes for each person who has the experience. We need this trial to help us learn how to improve the process for identifying high-risk patients and how to utilize both of these technologies better."
Dr. Schnall: “One size should not fit all in breast cancer screening, and the TMIST trial can show the way to a precision approach to screening. Look, we all know that 3D sees more lesions, but consequently, more women are having additional procedures, including biopsies and excisions. The question that TMIST asks is whether those huge expenditures of time, money, and psychological stress are worth it in terms of better outcomes for each person who has the experience. We need this trial to help us learn how to improve the process for identifying high-risk patients and how to utilize both of these technologies better."
There have been multiple published retrospective case series demonstrating that digital breast tomosynthesis (DBT) detects more breast cancer than planar digital mammography (DM). Do these results impact the importance of TMIST?
Dr. Schnall: “The answer to this question is a clear ‘no’. Among the largest series, the Population-based Research to Optimize the Screening Process (PROSPR) consortium demonstrated a cancer detection rate of 5.8/1,000 screens for DBT vs 4.4/1,000 for DM (Conant et al JAMA ONCOL 2019). In addition, the cancers detected by DBT were smaller and less likely to be associated with nodal metastasis. Although the PROSPR consortium and other series clearly demonstrate that DBT will detect more early cancer, they do not relate detection of these early cancers to better outcomes for the screening population.
What is the current status of the trial?
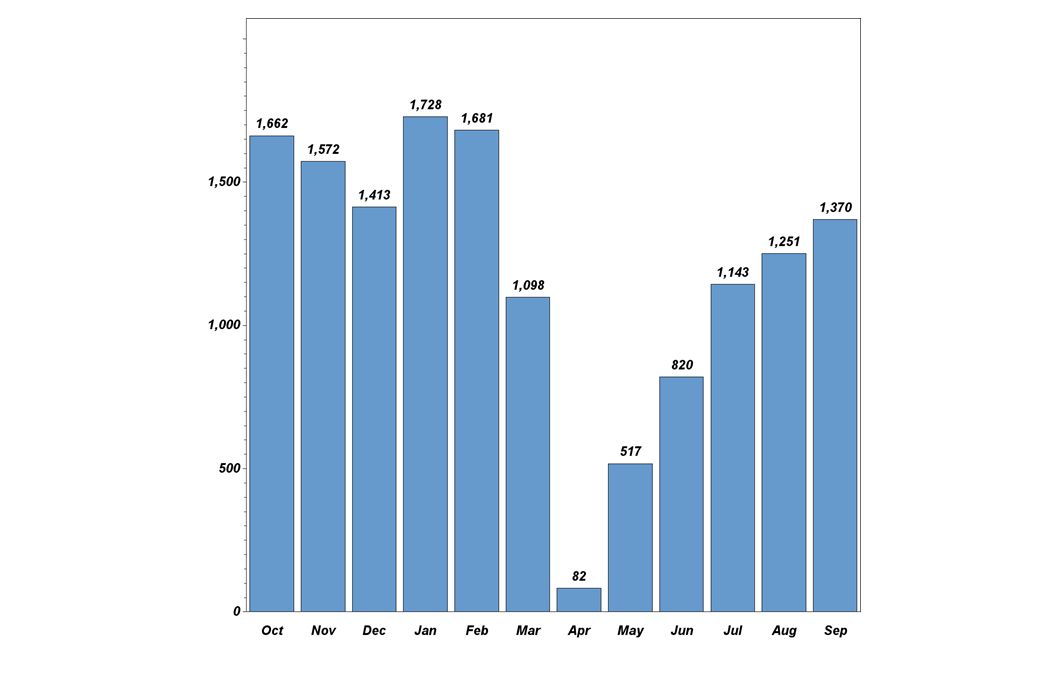
Dr. Pisano: "While the COVID pandemic did affect recruitment during the CDC-recommended spring and summer shutdown of cancer screening nationwide, the trial is recovering from that setback (see graph below). In the 14 months before the onset of the pandemic, TMIST enrollment quadrupled, and trial sites doubled. Recruitment rose to 1700 accruals by January 2020. Last month, TMIST sites recruited over 1300 women. We have approximately 50 more sites that are in the onboarding process now. Several more are in talks to join the trial. This is a great foundation. Patient accrual will accelerate as these sites become a member of ECOG-ACRIN or another clinical trial group (required to take part in NCI trials) and enter the study.
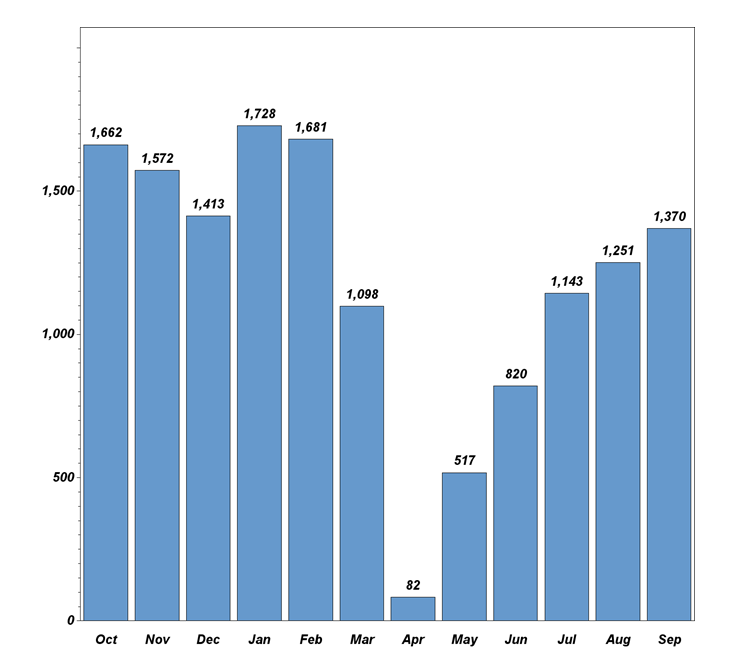
TMIST participant recruitment last 12 months.
October activity is ahead of September, with 1121 new enrollees at 77 sites—including six new sites—in the first 12 workdays. If the same recruitment rate continues for the last ten working days, the October enrollment will exceed 2000, making it the highest month on record. That stated, COVID has drastically changed the medical landscape. Patient accrual expectations are going to have to be reflective of the post-COVID shutdown reality. Readers may be aware that the NCI recently launched a review of TMIST accrual. It is important to note that the trial remains open and active during this review. With the current momentum, NCI leadership has specifically assured us that it will not close TMIST down (see statement below from the NCI director). ECOG-ACRIN leadership, Constantine Gatsonis (the study statistician), the trial scientific oversight groups (the TMIST steering committee, scientific advisory board, and advocacy committee), and I will be working together with NCI officials to improve the efficiency of TMIST so we can fulfill the study goals.”
In what ways do investigators plan to boost enrollment?
Dr. Pisano: Participating sites are pushing forward with site and patient recruitment as planned—and are doing well despite the constraints of the pandemic. Each site is tailoring strategies to make their particular populations aware of the opportunity to participate. The TMIST recruitment and patient materials are available here in Spanish, Chinese Simplified, and Korean. The Minority Underserved NCORPS are doing a great job with outreach and accrual—such that TMIST has enrolled at least twice as many Black and African-American women than the national average for clinical trials.
Statement from the National Cancer Institute
 "Clinical trials, studies that answer critical questions about cancer detection, prevention, and care, are only possible through collaborations between researchers, clinical care providers, and most importantly, patients," said Dr. Norman E. “Ned” Sharpless, NCI Director. "Due to a changing healthcare landscape, including challenges caused by the global COVID-19 pandemic, we have asked the NCI Clinical Trials Translational Research Advisory Committee to form a working group to provide general guidance regarding cancer screening trials, starting with TMIST. This group has recently been formed and the membership is posted on NCI’s website, cancer.gov.
"Clinical trials, studies that answer critical questions about cancer detection, prevention, and care, are only possible through collaborations between researchers, clinical care providers, and most importantly, patients," said Dr. Norman E. “Ned” Sharpless, NCI Director. "Due to a changing healthcare landscape, including challenges caused by the global COVID-19 pandemic, we have asked the NCI Clinical Trials Translational Research Advisory Committee to form a working group to provide general guidance regarding cancer screening trials, starting with TMIST. This group has recently been formed and the membership is posted on NCI’s website, cancer.gov.
"NCI supports the ECOG-ACRIN reactivation of the study sites that had been paused during the pandemic and the certification of additional sites," Sharpless continued. "Importantly, both NCI and ECOG-ACRIN remain committed to the women participating in the TMIST study, including those who have already enrolled, and we are grateful for their partnership."
Case Examples: How Sites Are Implementing TMIST
 TMIST Team at University of Cincinnati Medical Center Overcomes COVID-19 Challenges Read Article
TMIST Team at University of Cincinnati Medical Center Overcomes COVID-19 Challenges Read Article
 No Secrets about the Success of Enrolling African American Women at Boston Medical Center in TMIST Read Article
No Secrets about the Success of Enrolling African American Women at Boston Medical Center in TMIST Read Article
 Accruing to TMIST in Los Angeles’s Hispanic Community Read Article
Accruing to TMIST in Los Angeles’s Hispanic Community Read Article
 Carle Cancer Center and Gulf South Clinical Trials Network Adopt Different Approaches for Successful TMIST Accrual, with Tracy Sharp and Samantha Wright Read Article
Carle Cancer Center and Gulf South Clinical Trials Network Adopt Different Approaches for Successful TMIST Accrual, with Tracy Sharp and Samantha Wright Read Article
 The University of New Mexico Experience of Implementing TMIST Read Article
The University of New Mexico Experience of Implementing TMIST Read Article
 Direct Contact Boosts TMIST Recruitment at Spartanburg Site, with Tom Leucks, BSN-RN Read Article
Direct Contact Boosts TMIST Recruitment at Spartanburg Site, with Tom Leucks, BSN-RN Read Article
Learn more about TMIST (EA1151) at ecog-acrin.org.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
