
From the Co-Chairs, September 2021
September 30, 2021
Trial Spotlight: Elyse Park on EAQ171CD – the Smoke-Free Support Study 2.0
October 19, 2021News in Brief, October 2021

Register Now for the Virtual Fall 2021 Group Meeting
Registration is still open for the Virtual Fall 2021 Group Meeting, taking place online from Wednesday, October 20 – Friday, October 22. Please read the Group Meeting Tips and Highlights post in this issue.
EA Researchers Link Chronic Stress to Poor Breast Cancer Outcomes
A new study by researchers in the Cancer Control and Outcomes Program analyzed allostatic load and genetic ancestry data from the E5103 phase III clinical trial, one of the first large breast cancer treatment trials to assemble a biorepository and database of patient information for future research, including demographics and DNA (Miller KD. J Clin Oncol 2018). Prior studies suggested that allostatic load and genetic ancestry each play a role in poor breast cancer outcomes; however, no studies have looked at both factors at the same time in a study population.
"We observed that women with breast cancer who had a high allostatic load at the beginning of the study had a greater likelihood of stopping chemotherapy early and a higher risk of death," said lead investigator Samilia Obeng-Gyasi, MD, MPH (Ohio State University). "In contrast, we did not observe an association between genetic ancestry and survival or chemotherapy completion. This finding suggests that allostatic load may be better than genetic ancestry at predicting chemotherapy completion and overall survival."
This analysis received national attention when Dr. Obeng-Gyasi was featured on the press program for the 14th AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved in October. Patients in the trial had lymph node-positive or high-risk lymph node-negative, HER2-negative breast cancer.
NCI-MATCH Continues to Enroll Patients
The study chairs for this trial are Alice Chen, MD (NCI) and Keith Flaherty, MD (ECOG-ACRIN). The study co-chairs are Lyndsay Harris, MD (NCI) and Peter O’Dwyer, MD (ECOG-ACRIN).
Investigators and research staff, please continue to keep the open treatment arms top-of-mind when considering treatment options for your patients. Important updates:
- NCI-MATCH has eliminated the need for a formal referral letter from the laboratories, with activation of Addendum #29 on October 14
- Attendees of the Virtual Fall 2021 Group Meeting, mark your calendars for the NCI-MATCH and ComboMATCH Update and Education Session on Wednesday, October 20 from 9:30-10:30 AM Eastern Tme
- Arm Z1M (LAG-3 expression with MMR deficiency) accrual is temporarily suspended and will resume after the study team processes an amendment with updated safety information
Pancreatic Cysts ‘Almost an Epidemic’ Dr. Schnall Tells The Cancer Letter
The EA2185 pancreatic cyst surveillance trial is currently enrolling people with newly identified pancreatic cycts measuring 1 centimeter or greater, to determine the most effective surveillance strategy for these individuals. Recently, The Cancer Letter featured the trial in a cover story, Pancreatic cysts are common and usually benign—except those that turn deadly. (Vol. 47, No. 37).
The story is based on interviews with several ECOG-ACRIN leaders about the trial—and the challenges of conducting prevention and screening studies.
"There's almost an epidemic of pancreatic cystic lesions. I can tell you, because I do this for a living, the challenge is that the imaging studies are becoming so good that, inadvertently, we're finding pancreatic cysts are incredibly common, and we've got to get our arms around figuring out how to manage these," said Mitchell D. Schnall, MD, PhD, a radiologist at the University of Pennsylvania and group co-chair of ECOG-ACRIN. "Because every time somebody's getting a workup because of hematuria, we're looking at their kidneys. Every time somebody's getting a workup because of GI distress or because their biliary tract is being worked up, right, we're seeing the pancreas."
A conversation with Dr. Schnall and Peter O’Dwyer, also group co-chair of ECOG-ACRIN, appears here.
"Nobody, patients or doctors, want to operate on people who aren't going to benefit. By the same token, they don't want to withhold surgery from patients who will," said EA2185 study chair David S. Weinberg, MD, MSc (Fox Chase Cancer Center). "Our ability to risk-stratify needs to improve because missing the chance to prevent cancer is a terrible thing. On the other hand, subjecting someone to a major operation with major complications, at least potentially, is also a terrible thing. So, how to balance those two poles through a surveillance strategy is the question."
A conversation with Dr. Weinberg appears here.
Samilia Obeng-Gyasi Named AACR Faculty Scholar

The Minorities in Cancer Research Council of the American Association for Cancer Research (AACR) honored Samilia Obeng-Gyasi as a 2021 Minority and Minority-Serving Institution Faculty Scholar in Cancer Research. Recipients of this award are scientists who are working at the level of Assistant Professor or above who are engaged in meritorious basic, clinical, or translational cancer research.
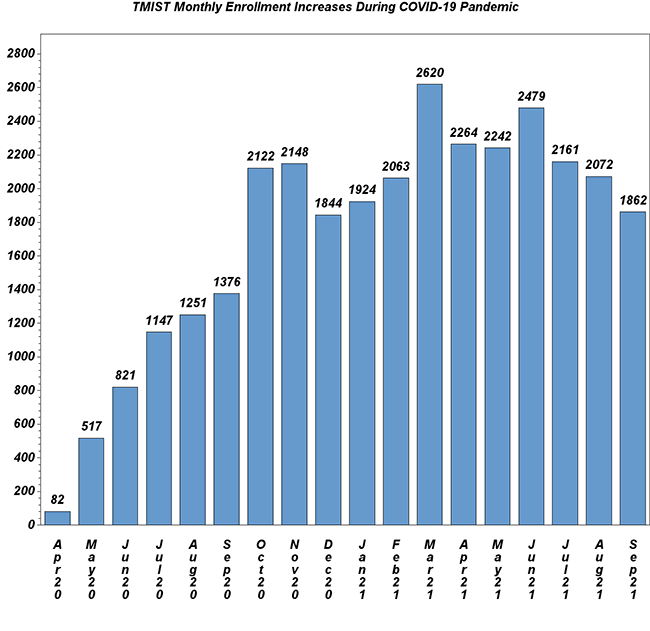
Amidst Ongoing Pandemic, TMIST Enrollment Remains Strong
The study chair for this trial is Etta Pisano, MD (Beth Israel Deaconess Medical Center, Harvard Medical School, and the American College of Radiology).
Amidst the ongoing COVID-19 pandemic, monthly accrual to the TMIST breast cancer screening trial continues at a steady pace (see below). Total enrollment stands at 56,874 women as of October 14. There are now 114 sites with at least one participant, a sign that new sites continue to join the trial. Important updates:
- At a recent National Cancer Institute (NCI) media briefing, TMIST study chair Etta Pisano, MD, stated that, despite the ongoing COVID-19 pandemic, TMIST has enrolled more patients than any other NIH-sponsored clinical trial since non-urgent care resumed last July
- A study amendment is in process to modify TMIST to more quickly complete patient accrual and reach the primary study endpoint
- Financial burden can be a barrier to TMIST participation, especially for women of color, and can be exacerbated by COVID-19. To better understand this barrier, a supplemental TMIST trial, EAQ201, will open in November to evaluate the effects of COVID-19 on the financial health of women who decline to participate in TMIST.
- New sites may join this trial! If your site is interested in offering this trial to your constituents, email TMIST@acr.org to discuss the study requirements, reimbursement/payment structure, and how to start the application process.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
