
From the Co-Chairs, November 2024
November 25, 2024
Trial Results: ECOG-ACRIN and PrECOG Research Round-Up
December 23, 2024News in Brief, December 2024

ECOG-ACRIN Advocates Tackle Translational Research
Inspired in part by the Cooperative Group leaders’ recent policy paper Correlative Science in the Cooperative Group System—Re-Engineering for Success, the members of the ECOG-ACRIN Cancer Research Advocates Committee are taking part in a training series dedicated to the subject of translational research. The series’ aim is to provide in-depth training to increase understanding and help individuals be better positioned to advocate for changes proposed in the publication. Improvements center around garnering funding through public-private partnerships and making regulatory changes to improve researchers’ access to biospecimens contributed by clinical trial participants for correlative studies.
The five-part series launched in December with talks by Dr. David Gerber of UT Southwestern/Simmons Comprehensive Cancer Center and ECOG-ACRIN group co-chair Dr. Peter O’Dwyer of the University of Pennsylvania/Abramson Cancer Center. It will conclude at the ECOG-ACRIN Spring 2025 Group Meeting in May.
EA8191/INDICATE Patient Video Series Now Available
A three-part patient educational video series explaining the EA8191/INDICATE trial is now available after being approved by the National Cancer Institute’s Central Institutional Review Board! The study, led by Dr. Neha Vapiwala (University of Pennsylvania/Abramson Cancer Center), is for patients with prostate cancer that has returned after surgery. The first video (below) provides an overview of the trial, while videos two and three share details about what happens in each of the two treatment groups. EA8191/INDICATE could help personalize treatment for men with post-prostatectomy biochemical recurrence and potentially improve progression-free survival. Watch the videos now to learn more.
ECOG-ACRIN Training Video Resource Library
 Recently, ECOG-ACRIN launched a new video resource library of help topics to support site personnel, including nurses, clinical research associates and coordinators, data managers, pharmacists, and others. Short videos on a variety of topics, from patient registration to sample management to preparing for a pharmacy audit, are now available for site staff to use as a quick reference or to supplement their knowledge base. More videos are in development and will be added on a rolling basis. View the ECOG-ACRIN Training and Resources channel on YouTube.
Recently, ECOG-ACRIN launched a new video resource library of help topics to support site personnel, including nurses, clinical research associates and coordinators, data managers, pharmacists, and others. Short videos on a variety of topics, from patient registration to sample management to preparing for a pharmacy audit, are now available for site staff to use as a quick reference or to supplement their knowledge base. More videos are in development and will be added on a rolling basis. View the ECOG-ACRIN Training and Resources channel on YouTube.
TMIST Update
The study chair for this trial is Etta D. Pisano, MD (American College of Radiology).
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is reaching its enrollment goal of 108,805 women. The trial will close to new accruals on December 20, 2024. Learn more about TMIST and access the latest newsletter for clinical sites and educational resources on ecog-acrin.org.
Fall Group Meeting Session Recordings Still Available
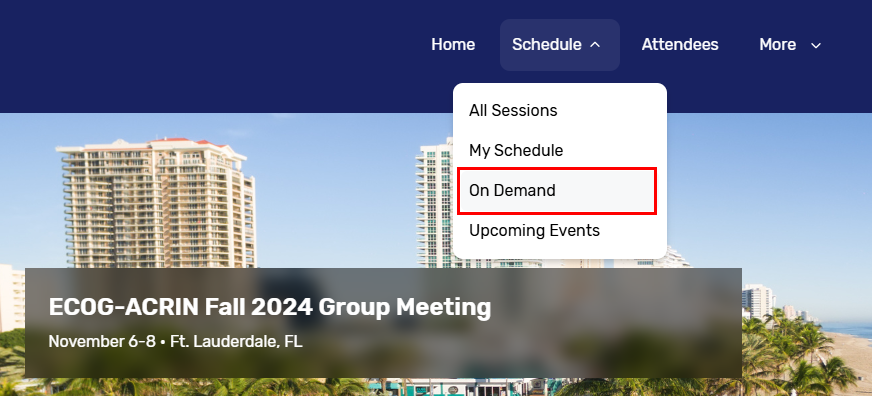
Did you miss the Fall 2024 Group Meeting? If you are an ECOG-ACRIN member, you can catch up on important study updates and trainings by viewing our library of session recordings. These are available for both attendees and those who missed the meeting. Attendees can simply visit the Attendee Hub Website and log in with the information provided during registration. Everyone else should first register for the meeting and then visit the Attendee Hub to view session recordings on demand.
After registration, users should go to the Attendee Hub and log in with the information they provided. Navigate to ‘Schedule,’ and then select ‘On Demand’ from the dropdown list. Note: The Attendee Hub Website will expire 90 days following the meeting (early February 2025).
In the coming weeks, we will also post select session recordings and resources from the Fall Group Meeting on the members’ section of the ECOG-ACRIN website. These materials will be available to anyone with member login credentials, regardless of meeting attendance.
ASCO 2025 Abstract Submission Deadline Approaching
The American Society of Clinical Oncology (ASCO) is accepting abstract submissions for the 2025 Annual Meeting through Tuesday, January 28, 2025, at 11:59 PM EST. More information is on the ASCO website. Please notify publications@ecog-acrin.org immediately if you plan to submit an abstract to ASCO.
All draft abstracts using ECOG-ACRIN study data must be submitted to publications@ecog-acrin.org by Wednesday, January 15, 2025, including trial-in-progress abstracts and late-breaking abstract placeholders. This deadline is firm and necessary to ensure sufficient time for ECOG-ACRIN to prepare/review the authorship line and for a courtesy review by NCI and industry collaborators, if applicable.
For questions regarding ECOG-ACRIN publication procedures, please contact the ECOG-ACRIN publications team. ECOG-ACRIN publication and authorship guidelines are available on the member section of the ECOG-ACRIN website.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
