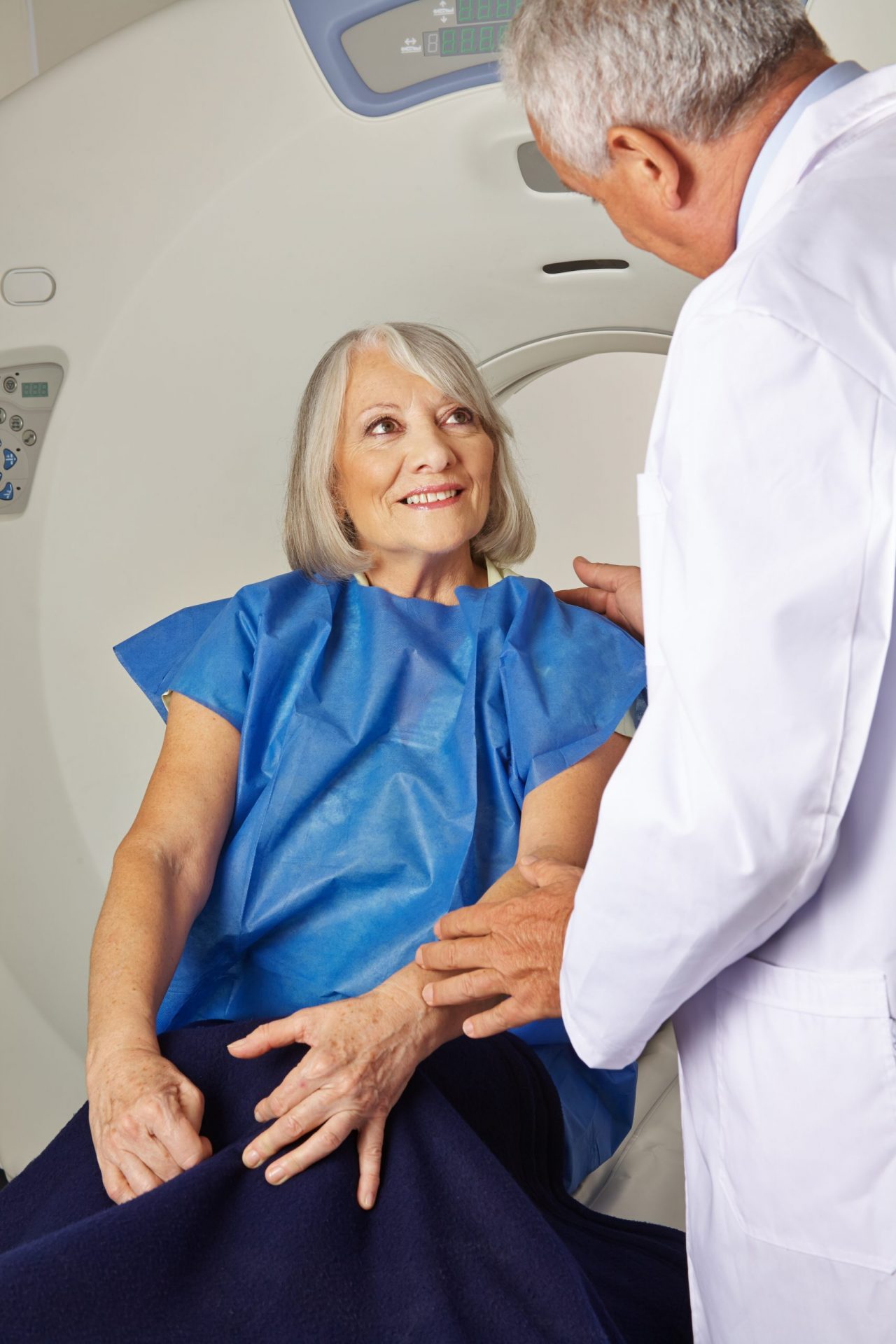
Now Enrolling: EA2185 to Compare the Clinical Impact of Pancreatic Cyst Surveillance Programs
February 28, 2020
Trial Spotlight: E1Q11 / EROS For Female Cancer-Diagnosed Patients with Reproductive Health Goals
April 15, 2020From the Co-Chairs, February 2020


By Peter J. O’Dwyer, MD (left), and Mitchell D. Schnall, MD, PhD
Following upon our successful grant awards for our ECOG-ACRIN research, the Cooperative Group Chairs were invited to meet with National Cancer Institute (NCI) Director Dr. Norman (Ned) Sharpless, along with Drs. Doroshow and Mooney, at Dr. Sharpless's office in Bethesda in early January. The discussion centered on the role of the Groups in helping to advance the national cancer research agenda, and the meeting affirmed the significant contributions of the Groups in the past several years. In addition, there was acknowledgement that the alignment of goals between the NCI and the Groups was close, and that our shared sense of purpose made for the development of innovative and impactful trials in the public system. Nonetheless, we recognized that there are areas of mutual function, principally relating to speed of development at various levels, and access to samples for translational studies, which if improved, would make Cooperative Group research more attractive both for researchers and for pharma. In a follow-up document from the Group Chairs, two areas for future dialogue were identified: structural aspects of operations, and attention to the concept of cooperative development. Ideas have already been exchanged to prepare groundwork for these discussions.
Helpfully, in late December Congress passed and enacted an appropriations bill providing the NCI with a budget increase of $297 million for fiscal year (FY) 2020. This was part of an overall National Institutes of Health (NIH) budget increase of $2.6 billion. In a recent blog post, Dr. Sharpless explained that this will allow the NCI to extend current paylines, fund more than 125 additional competing awards in 2020 (compared to 2019), and fully restore commitments to noncompeting grants. Dr. Sharpless noted that they will allocate more than $210 million to support extramural research and training opportunities at universities, medical centers, and other institutions throughout the United States. Thanks to this budget increase, organizations like ours that rely on government funding can continue building momentum and making progress. In this issue, we highlight two trials: EA2185, recently activated, and EA3161, in progress. EA2185 is a test of approaches to the monitoring of incidentally found pancreatic cysts, a common finding in abdominal CT scans. Two approaches may be taken in monitoring the growth of these abnormalities, both credentialed by professional societies, one more intensive than the other. Neither has objective data to support its being a superior approach, and most important, there are few metrics that would predict that a given cyst might have a "bad biology." The differences between these standard approaches also potentially amount to billions per year, providing additional reasons to analyze outcomes. We hope that there will be broad interest in accrual to this trial. EA3161 has therapeutic potential in HPV-positive head and neck cancer - it tests the addition of nivolumab (vs. control) after definitive chemoradiation in patients with locally advanced disease. Clearly a successful result here would have the potential to enhance cure rates in this disease. Finally, we want to provide advance notice of the Second Robert L. Comis Translational Science Symposium. This will take place at our Spring Group Meeting on Wednesday, April 29 from 1:00 - 3:00 pm. The focus of the Symposium follows upon the initial Symposium at our last meeting, and will address the many research opportunities afforded by an emphasis on Big Data - for this meeting, on the generation of Real-World Evidence. As you are aware, for both therapeutic and imaging trials, a successful Phase Ill study is often the end of that particular research thread. A novel regimen or test, if approved by the FDA, is then implemented in the community without a real-time assessment of how well the novel approach contributes to health. Opportunities for analysis of treatment delivery, test application, benefits, toxicity, and specificity for patient subpopulations are clear, and are of interest both to advocacy groups as well as to the FDA, all through collection of real-world data. Friends of Cancer Research and the FDA have collaborated on guidelines for this research, and will be a key part of this symposium. We invite your participation, and especially will appreciate if you can reach out to researchers who may be particularly interested in such trials. Read the February 2020 issue here.![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
