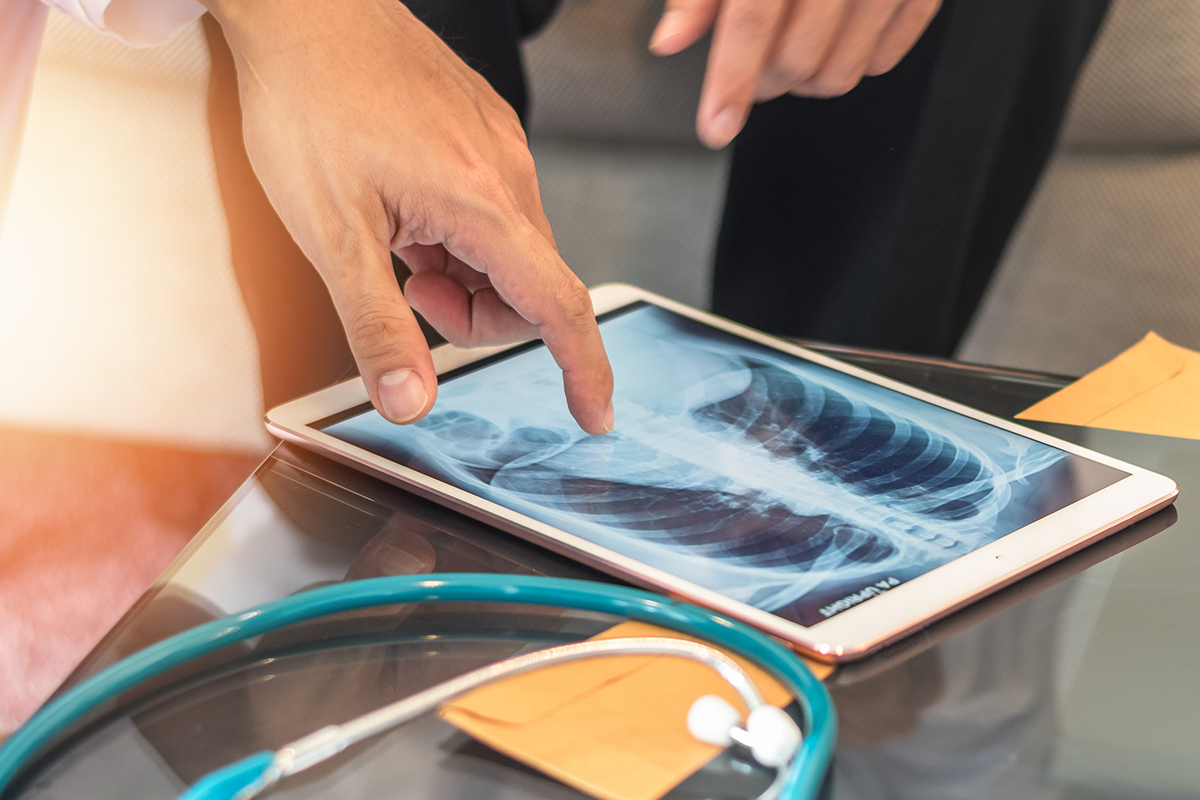
Trial Spotlight: Helena Yu Gives an Update on the EA5182 Trial for EGFR-Mutant Non-Small Cell Lung Cancer
March 7, 2024
Now Enrolling: EAQ222CD/CostCOM to Reduce Financial Hardship for Patients with Cancer
March 7, 2024ECOG-ACRIN Welcomes Catherine Handy Marshall as its First Winn Clinical Investigator Leadership Awardee

ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) and the other national cancer cooperative groups have partnered with Conquer Cancer®, the ASCO Foundation and Bristol Myers Squibb to support a new opportunity under the Robert A. Winn Diversity in Clinical Trials Award Program: the Winn Clinical Investigator Leadership Award (Winn CILA).
The CILA is a three-year career and leadership award for selected clinical cancer researchers who previously completed the two-year Winn Career Development Award Program (Winn CDA). The CILA is designed to provide advanced clinical trials knowledge, leadership skills, mentorship, and sponsorship to succeed as independent clinical researchers, team members, and leaders.
The first cohort of CILA recipients was announced in December 2023 and includes five physicians (referred to as Winn Investigators) who come from a range of oncology backgrounds and expertise. Each physician was matched with an NCI National Clinical Trials Network (NCTN) Group and paired with a mentor who is an experienced clinical trialist. The overall goal is for each Winn Investigator to acquire the skills to effectively develop, implement, and oversee a clinical trial sponsored by the NCTN cooperative group.
 ECOG-ACRIN is delighted to have Catherine Handy Marshall, MD, MPH (pictured), as its first Winn Investigator. Dr. Marshall is an assistant professor of oncology at Johns Hopkins University’s School of Medicine and a medical oncologist at the Sidney Kimmel Comprehensive Cancer Center located in The Johns Hopkins Hospital. Her clinical practice and research focus on prostate cancer. She also has a strong research interest in cardiovascular disease, which she studied during her residency in internal medicine, and the relationship between cardiovascular disease and cancer.
ECOG-ACRIN is delighted to have Catherine Handy Marshall, MD, MPH (pictured), as its first Winn Investigator. Dr. Marshall is an assistant professor of oncology at Johns Hopkins University’s School of Medicine and a medical oncologist at the Sidney Kimmel Comprehensive Cancer Center located in The Johns Hopkins Hospital. Her clinical practice and research focus on prostate cancer. She also has a strong research interest in cardiovascular disease, which she studied during her residency in internal medicine, and the relationship between cardiovascular disease and cancer.
Dr. Marshall received her undergraduate degree from Harvard University and her medical degree from Johns Hopkins University, where she also completed her residency and an oncology fellowship. She took a year off from medical school to complete a master’s degree in public health, with a focus on health systems and policy. During her oncology fellowship, she developed an interest in prostate cancer—a disease that has affected several of her family members, and one that is more prevalent in African American and Black communities.
Dr. Marshall’s recent research involves the genetics of prostate cancer and prostate cancer outcomes, as well as how gene alterations predict response to treatment. Her Winn CDA award was focused on a trial of olaparib, in the absence of androgen deprivation therapy, for men with and without genetic alterations in prostate cancer. Additionally, she is studying clonal hematopoiesis, a risk factor for heart disease, and how it may be relevant to prostate cancer. Her mentor at ECOG-ACRIN is Gary I. Cohen, MD, former chair of the Community Cancer Committee, former member of the Executive Committee, and current community co-chair of the Melanoma Committee. Dr. Cohen, now working in a consulting capacity, is the previous director of the Sandra and Malcolm Berman Cancer Institute at Greater Baltimore Medical Center. He holds an appointment as associate professor in the Department of Oncology at the Kimmel Cancer Center at Johns Hopkins.
“We are thrilled to welcome Dr. Marshall and are pleased by her selection for funding by the Winn CILA Award to advance her clinical trials and leadership skills within ECOG-ACRIN,” said Dr. Cohen. “She has already demonstrated remarkable progress as a physician-scientist and we expect further rapid career achievement as she participates, develops, and leads clinical trial efforts in her chosen areas of interest.”
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
