
From the Co-Chairs, May 2020
May 29, 2020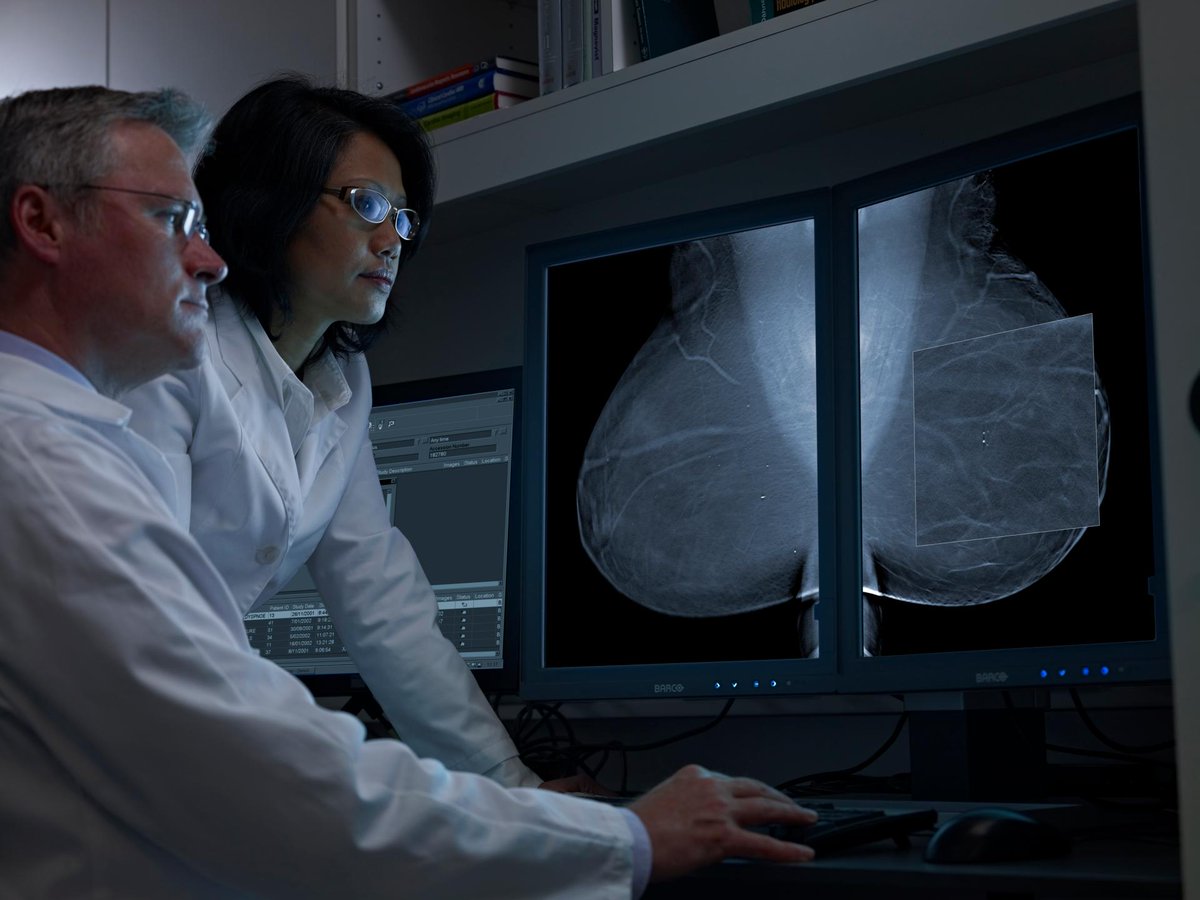
The Value of TMIST Compared to Other Breast Cancer Screening Studies
June 10, 2020ECOG-ACRIN Research at #ASCO20: How Twitter Helped Fill the Virtual Void
 This year’s ASCO Annual Meeting was a significant one for ECOG-ACRIN. EA investigators shared a wide range of research results, presenting findings from eight different clinical trials, including late-breaking data in two plenary presentations. The meeting was also significant in another key way – not just for EA, but for all attendees. It took place entirely online.
This year’s ASCO Annual Meeting was a significant one for ECOG-ACRIN. EA investigators shared a wide range of research results, presenting findings from eight different clinical trials, including late-breaking data in two plenary presentations. The meeting was also significant in another key way – not just for EA, but for all attendees. It took place entirely online.
This new virtual format, developed out of necessity, yielded some interesting effects. One, in particular, was an increase in social media activity, likely resulting from the loss of face-to-face interactions. According to an article in Medscape, this year’s meeting generated almost 18K tweets compared to approximately 15K tweets last year, and this despite 300 or so fewer users contributing to the conversation.
What did some of these conversations look like? Consider those sparked by the two ECOG-ACRIN plenary presentations. In LBA2, lead investigator Seema A Khan, MD (Northwestern University) shared the results of E2108, which found that surgeryand radiation after initial systemic therapy do not improve overall survival for women who first present with metastatic breast cancer. In LBA3, lead investigator Shaji K. Kumar, MD (Mayo Clinic) presented data from the ENDURANCE/E1A11 trial, showing that the drug carfilzomib does not improve outcomes in newly diagnosed myeloma compared to bortezomib.




One could argue that, this year, social media played a more critical role than usual, fostering discussions and debates that simply could not happen in person. However, most seemed to feel that social media was an imperfect substitute. The sentiment behind Dr. Fonseca’s statement above, “TBH I wish Shaji could have done in person…” was echoed by many others.


At least, as Dr. Hamilton notes above, “there was no sacrifice of science,” and the oncology community found a way forward through challenging circumstances. That in itself was no small feat, and all those affected by cancer will be better for it. In addition to the two plenary presentations, EA also shared findings from two TAILORx sub-studies, head and neck cancer trial E3311, lung cancer trial EA5161, arm Z1F of the NCI-MATCH trial, and the cancer care delivery study COMET. Learn more about the results of these trials.
Also of note, PrECOG, LLC reported positive results from mesothelioma trial PrE0505. Led by Patrick Forde, MD (Johns Hopkins), the study found that adding durvalumab to standard chemotherapy improved overall survival in mesothelioma. PrECOG is a cancer research group formed in 2006 by the ECOG Research and Education Foundation, Inc. Learn more about PrE0505.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
