
News in Brief, September 2021
September 30, 2021
Should Participants Know the Arm to Which They Were Assigned in a Randomized Clinical Trial?
September 30, 2021Trial Spotlight: Selina Luger on the PrE0905 Study for FLT3 Mutated Acute Myeloid Leukemia

Randomized Trial of Gilteritinib vs. Midostaurin in FLT3 Mutated Acute Myeloid Leukemia
 By Selina M. Luger, MD, Study Chair
By Selina M. Luger, MD, Study Chair
Approximately one-third of patients with acute myeloid leukemia (AML) have a FLT3 mutation—and the presence of a FLT3-ITD mutation has been shown to increase the likelihood of relapse and decrease survival. The tyrosine kinase inhibitor (TKI) midostaurin has demonstrated promise, improving survival in patients with FLT3 mutated AML receiving induction chemotherapy. However, the doses of midostaurin that are feasible are not thought to optimally inhibit FLT3. Better FLT3 inhibitors are needed. Thus, PrE0905, an open-label randomized phase II study, is exploring the potent FLT3 inhibitor gilteritinib.
Gilteritinib was previously approved for patients with relapsed/refractory FLT3-mutated AML after clinical trials demonstrated its effectiveness in improving survival as a single agent in this setting. It is not yet approved in the upfront setting. PrE0905 is studying the effects of gilteritinib compared to midostaurin when added to AML induction therapy with daunorubicin (90 mg/m2/d x 3 days) and cytarabine (100 mg/m2/d x 7 days).
Adult patients who are newly diagnosed, have not yet received treatment, and are age 70 or younger are eligible for this trial. They must have AML with a FLT3 mutation. Patients with a known core binding factor or with acute promyelocytic leukemia (with or without a FLT3 mutation) are not eligible. The study’s primary endpoint is rate of FLT3 mutation negative composite complete remission after induction. Patients may choose to remain on study for consolidation therapy for up to 4 cycles of high dose cytarabine with gilteritinib vs. midostaurin.
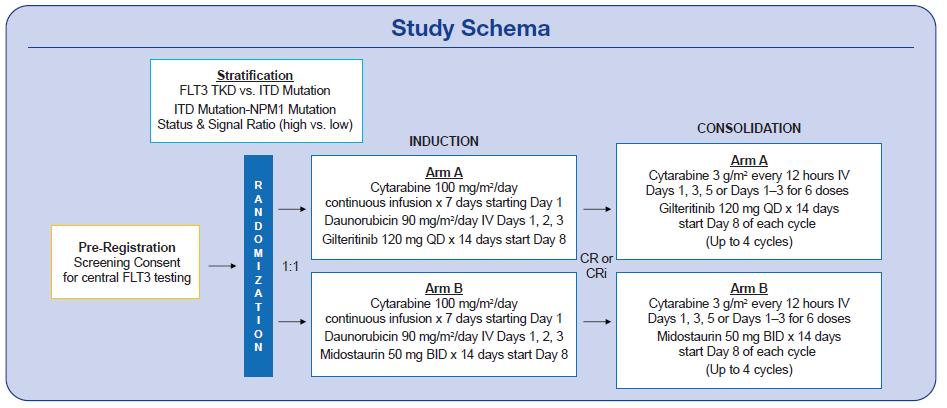
In order to be eligible for participation, a patient’s sample must be sent centrally for FLT3 mutation analysis as well as additional pre-randomization studies. Sites may send a peripheral blood sample if bone marrow is not obtainable and adequate numbers of circulating blasts are present. A pre-screening consent form is signed at the time of sample collection so that any patient undergoing a bone marrow biopsy for possible leukemia can have a sample sent. The full study consent does not need to be signed until full registration/randomization.
With amendment #2 approved, participants may now start the study-specified induction (cytarabine 100 mg/m2/d + daunorubicin 90 mg/m2/d) while they wait for the central FLT3 mutation results. Repeat testing for FLT3 mutation is required at response assessment after induction in all patients as well as after first consolidation for those patients who remain on study.
PrE0905 is currently open at 41 sites across the US, and the study has enrolled and randomized 80/179 patients.
Learn more about the PrE0905 trial at clinicaltrials.gov, or hear Dr. Luger describe the study in the informational video below (intended for physicians and site staff). The video includes details on the patient screening process and procedures for submitting samples for FLT3 testing.
Sites that are interested in participating in the study should contact PrECOG.
About PrECOG
PrECOG, LLC is a cancer research group formed as a not-for-profit limited liability company in 2006 by the ECOG Research and Education Foundation, Inc. It operates outside of the National Cancer Institute’s federal funding structure, known as the National Clinical Trials Network. A central focus of PrECOG is to support the overall scientific research goals of the ECOG-ACRIN Cancer Research Group. For further information, please visit www.ecog-acrin.org, and follow us on Facebook and Twitter @PrECOGonc and @EAonc.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
