
A New Guide Encourages the Use of Language that is Respectful of Patients, Free of Stigma, Inclusive, and Equitable
September 30, 2021
Now Enrolling: Phase II of EA9152 for Acute Lymphoblastic Leukemia
September 30, 2021NCI-MATCH Eliminates the Need for a Formal Referral Letter from the Laboratories
The ECOG-ACRIN Cancer Research Group’s operations office pre-activated Addendum #29 in the NCI-MATCH (EAY131) cancer precision medicine trial on September 17, 2021. Site personnel may now begin processing the amendment locally in preparation for the formal activation on October 14, 2021.
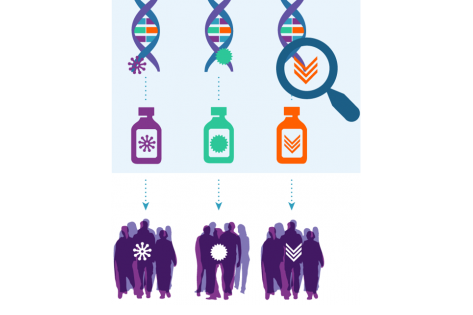
While Addendum #29 contains numerous changes, perhaps the most significant revision aims to help oncologists determine more quickly whether their patients are eligible for one of the treatment subprotocols. In short, they no longer need a formal referral letter or email from the lab to enroll their patient to the trial’s Step 0 for an eligibility determination.
NCI-MATCH is open to patients with cancer who receive tumor gene testing at cancer centers and community hospitals participating in the trial. Nearly 1100 clinical sites in every state, the District of Columbia, and Puerto Rico are currently participating in MATCH.
The process for identifying patients for the trial begins after a physician at a participating trial site orders routine genomic sequencing from one of the designated commercial labs to guide clinical care for their patient. The lab looks for tumor abnormalities being studied in NCI-MATCH as part of their standard procedure.
Previously, if the lab found a match, it was required to send a formal referral to the ordering physician (either a letter or email). Then, oncology teams needed to submit that document when enrolling the patient to Step 0 for a screening assessment. Thus, sites that reviewed patients’ genomic tests and identified sequencing matches on their own still needed to wait for a formal notice from the lab. In these cases, the formal notification was a rate-limiting step to enrolling the patient to Step 0 to confirm eligibility.
Now, the physician uses the laboratory sequencing result to register the patient to Step 0 for a screening assessment. After that, the rest of the eligibility determination process is the same.
The goal of eliminating the formal referral is to see an uptick in registrations to Step 0, resulting in more patients assigned to the available treatment arms.
As a reminder to physicians and staff at current sites, please keep the nine open treatment arms top of mind when looking for treatment options for patients. Among these treatment options are the following tumor abnormalities:
- BRAF V600E or V600K mutations
- cKIT mutations; PDGFRA or PDGFRB variants and fusions
- EGFR activating mutations
- EGFR T790M (with/without an activating mutation) or rare activating mutations of EGFR
- LAG-3 expression with MMR deficiency
- MET amplification
- MET exon 14 deletion
- mTOR, KEAP1 or NFE2L2 mutations
- NTRK fusions
- PTEN mutations (except for a Copy Number=0) and PTEN negative by IHC
Is your site interested in opening NCI-MATCH? We encourage more NCI-affiliated sites to open the trial. Watch the video below to hear from sites about the benefits of participating in NCI-MATCH, plus an update from ECOG-ACRIN Study Chair Keith T. Flaherty, MD (Massachusetts General Hospital Cancer Center). Interested sites, please email match@jimmy.harvard.edu.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
