
From the Co-Chairs, July 2020
July 29, 2020
2020 Young Investigator Symposium (A Virtual Event)
September 14, 2020Trial Spotlight: Ingrid Mayer on Study EA1131 for Triple-Negative Breast Cancer, Including Men

Photo credit: Vanderbilt University Medical Center
Update on March 30, 2021: Study EA1131 is now closed to patient enrollment.
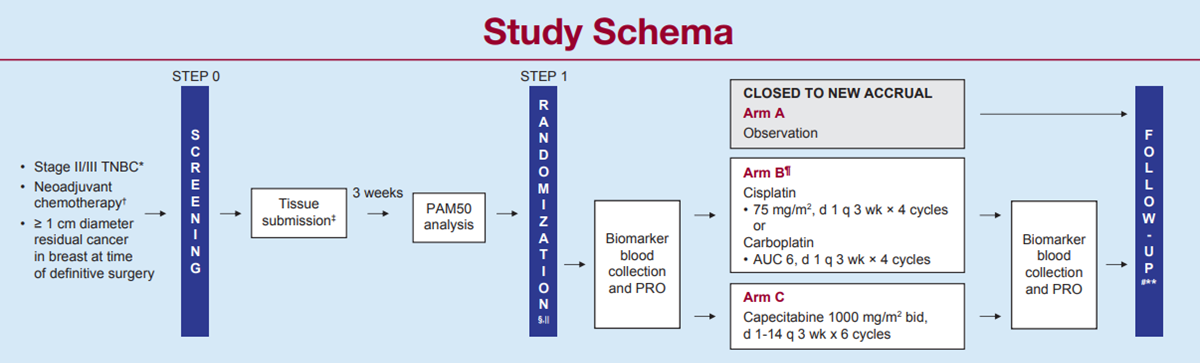
Platinum-Based Chemotherapy or Capecitabine in Treating Patients with Residual Triple-Negative Basal-Like Breast Cancer following Neoadjuvant Chemotherapy

By Ingrid A. Mayer, MD, MSCI
If you treat breast cancer patients, you know that patients with stage II or III triple-negative breast cancer (TNBC) have a high risk of developing metastatic recurrence within the first five years of their diagnosis. However, those who receive neoadjuvant chemotherapy (NAC) and achieve a pathologic complete response (pCR) at the time of surgery typically have great long-term outcomes. These patients do not need any further treatment than what they have already received and consequently can be spared the added toxicity of adjuvant therapy.
Conversely, patients with TNBC who receive NAC and DO NOT achieve a pCR will typically have poor long-term outcomes. These patients benefit from additional adjuvant treatment to prevent metastatic recurrence of their cancer. One such treatment already exists: post-operative capecitabine has been shown to improve disease-free survival (DFS) (70% alive at five years) and overall survival (OS) (79% alive at five years) in patients with TNBC and residual disease after conventional anthracycline-taxane based chemotherapy. But can we do better?
About 80% of TNBC, when subtyped with the PAM50 (Prosigna®) signature, are basal-subtype. Basal-subtype TNBC has a more aggressive biological behavior and has a dependency on DNA-damage genes/ proteins. Therefore, basal-subtype TNBC may have a better response to DNA-damaging agents such as platinum-based chemotherapy. Indeed, when added to NAC, platinum agents increase rates of pCR. However, temporary and permanent toxicity rates are quite high, and long-term benefit to outcome is still unclear, as these trials were not powered to definitively detect either RFS or OS advantage.
With this design, we are not committing all patients with TNBC to more chemotherapy upfront and therefore sparing patients who achieve a pCR the unnecessary added toxicity. Importantly, patients have the choice of starting treatment immediately after surgery or after completion of radiation (if applicable) and are able to co-enroll on other adjuvant studies, such as S1418, once their EA1131 chemotherapy is completed. Help us answer this important question as soon as possible. You can make a difference: Start accruing today!
Learn more about study EA1131 at ecog-acrin.org.
Dr. Mayer (Vanderbilt University) is the lead investigator for this trial.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
