
EA Launches Anti-Racist Agenda with Health Equity Inaugural Meeting
July 29, 2020
Now Enrolling: EA2186 / GIANT for Advanced Pancreas Cancer in Older Adults
July 29, 2020Trial Results: E3311 Leads to Phase III Trial in Head and Neck Cancer
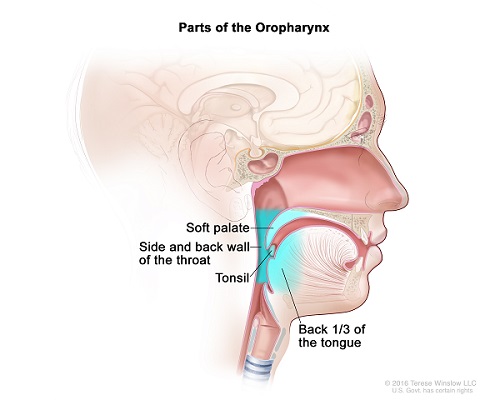
Created for the National Cancer Institute, http://www.cancer.gov
ECOG-ACRIN to Conduct Randomized Phase III Trial Based on Recent Results of Phase II Study E3311
The overall intent of E3311, which validated a less intense treatment for certain patients with human papillomavirus-positive (HPV+) throat cancer, was to gather essential data for the design of a future, randomized phase III trial. E3311 met its primary objectives. The first objective was to determine the feasibility of a prospective multi-institutional study of transoral robotic surgery (TORS) for HPV+ oropharynx cancer, followed by risk-adjusted adjuvant therapy. The second objective was to assess the oncologic efficacy following transoral resection and adjuvant treatment in patients determined to be at “intermediate risk” after surgical excision. To evaluate the latter, the study team reviewed the two-year progression-free survival rate.
 “For intermediate-risk patients -- those with uninvolved surgical margins, less than five involved nodes, and less than 1mm extranodal extension -- reduced-dose postoperative radiation therapy without chemotherapy appears sufficient ... this group had better outcomes than the group on usual high-dose radiation plus chemotherapy,” said lead investigator Robert L. Ferris, MD, PhD (UPMC Hillman Cancer Center), pictured left.
“For intermediate-risk patients -- those with uninvolved surgical margins, less than five involved nodes, and less than 1mm extranodal extension -- reduced-dose postoperative radiation therapy without chemotherapy appears sufficient ... this group had better outcomes than the group on usual high-dose radiation plus chemotherapy,” said lead investigator Robert L. Ferris, MD, PhD (UPMC Hillman Cancer Center), pictured left.
Dr. Ferris presented the results at the American Society of Clinical Oncology virtual annual meeting in May. These findings confirmed that the study’s patient stratification identified low- and intermediate-risk patients well, preserving their throat function and sparing them unnecessary short- and long-term toxicities.
 “ECOG-ACRIN now plans to pursue the current data with a randomized phase III trial of TORS-based treatment de-intensification compared with conventional chemoradiation,” said ECOG-ACRIN Head and Neck Committee Chair, Barbara A. Burtness, MD (Yale University), pictured left.
“ECOG-ACRIN now plans to pursue the current data with a randomized phase III trial of TORS-based treatment de-intensification compared with conventional chemoradiation,” said ECOG-ACRIN Head and Neck Committee Chair, Barbara A. Burtness, MD (Yale University), pictured left.
![ECOG-ACRIN logo[19516]275×75](https://blog-ecog-acrin.org/wp-content/uploads/2021/03/ECOG-ACRIN-logo19516275x75.png)
